[ad_1]
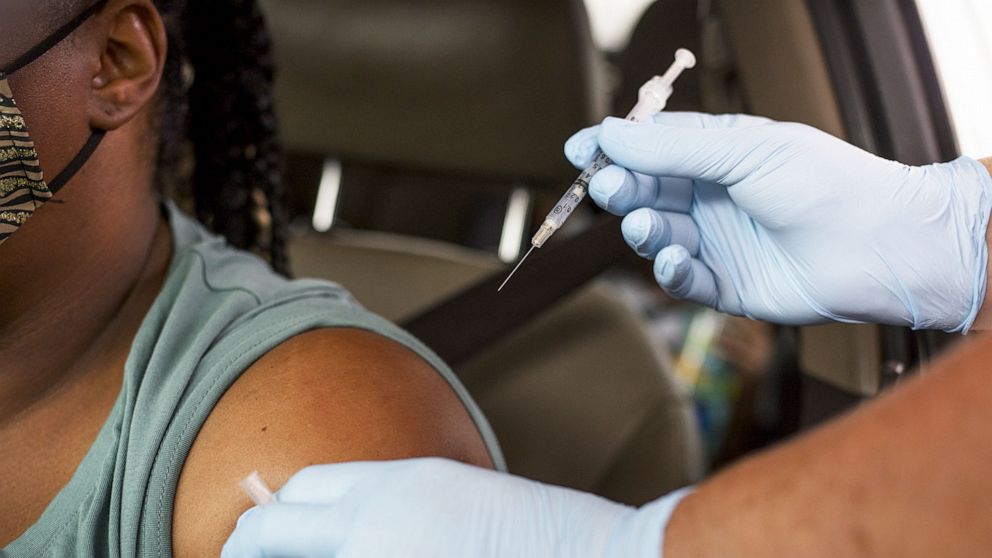
Over the next few weeks, the U.S. Food and Drug Administration is expected to recommend a plan for immunocompromised Americans to be vaccinated against COVID-19, a senior government official familiar with the planning of the day told ABC News on Thursday. ‘agency.
The plan is expected in early September, if not sooner, the government official said.
The timeline was first reported by The Wall Street Journal.
Many immunocompromised Americans have not had high immune responses to vaccines, leaving them vulnerable to the virus even after receiving an injection. The response has been poor, especially in transplant recipients, cancer patients, or people taking drugs that suppress their immune response.
Experts from the Centers for Disease Control and Prevention’s advisory group last month broadly supported giving these people a third dose to boost their immunity and called on the FDA to act on the issue.
Booster injections for the general population, which may be available at any given time, may not be part of the initial priority clearance from the FDA. The CDC and the FDA argue that there is not enough data to show that immunity has waned enough in those vaccinated to warrant a third injection for everyone – yet.
But booster shots for the immunocompromised have become an obvious priority.
Asked by ABC News Thursday during a White House COVID response briefing when people in that group could expect approval of a third shot, Dr.Anthony Fauci, White House chief medical adviser , said the boosters “will be implemented as quickly as possible”.
“It is extremely important for us to act so that these individuals receive their boosters. And we are working on it now, and we will make sure that it is implemented as quickly as possible. Because for us and for the individuals involved, it’s a very high priority, ”Fauci said.
Fauci also privately told governors during the National Governor’s Association’s appeal on Tuesday that people with compromised immune systems will be No.1 in the queue for recalls, and that the FDA is “working on a mechanism” to secure recalls. additional doses for this group “as soon as we can,” according to a reading of the call obtained by ABC News.
He also said he expected the general population to follow suit eventually.
“I think we’ll all go in that direction eventually,” Fauci said, without specifying when.
On Tuesday, FDA chief vaccine officer Dr Peter Marks said he expected to issue clearer guidance on recalls “by September.”
“I suspect, again, by September, that we will be able to make a more coherent statement on what the recommendation will be here,” Marks said.
Marks said it would not be a general roadmap, but would need specific instructions for certain groups of people and depend on the blows originally received.
“We realized we couldn’t just have a recommendation for a vaccine because it couldn’t just be, ‘Well I got vaccine X, what should I do? We need to have a recommendation for everyone who has been vaccinated, ”Marks said.
For Phil Canuto, who remained immunocompromised after a kidney transplant 19 years ago, the news of the recalls on the horizon has thrilled him.
Canuto, from Akron, Ohio, had no immune response after two doses of the Pfizer vaccine. The growing spread of the delta variant and data showing that even people who have been vaccinated can spread the virus have put him and his family members on high alert.
“If FDA approval for additional injections is imminent, I am delighted,” he said in a message to ABC News.
“I know a lot of transplant patients have tried to surreptitiously get additional injections, lying to contextual vaccination sites about their previous injections,” he said, adding that this news would give patients the opportunity to make medically informed decisions with their physicians. .
[ad_2]
Source link