
[ad_1]
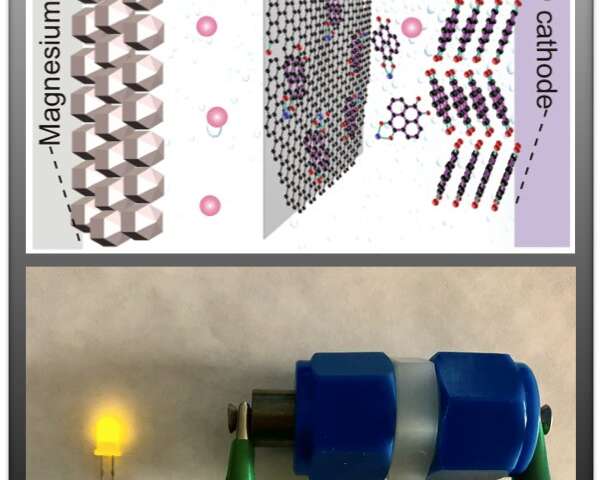
Researchers at the University of Houston and the Toyota Research Institute of North America have reported a breakthrough in the development of magnesium batteries, allowing them to provide a power density comparable to that of lithium-ion batteries. Credit: University of Houston
Magnesium batteries have long been seen as a potentially safer and less expensive alternative to lithium-ion batteries, but previous versions were severely limited in the power they delivered.
Researchers from the University of Houston and the Toyota Research Institute of North America (TRINA) report in Energy of nature that they developed a novel cathode and electrolyte – previously the limiting factors for a high energy magnesium battery – to demonstrate a magnesium battery capable of operating at room temperature and delivering a power density comparable to that offered by lithium-ion batteries.
As the need for grid-scale energy storage and other applications grows more pressing, researchers have been looking for less expensive and more readily available alternatives to lithium.
Magnesium ions hold twice the charge of lithium, while having a similar ion radius. As a result, the dissociation of magnesium from electrolytes and its diffusion into the electrode, two essential processes that take place in conventional intercalation cathodes, are slow at room temperature, leading to low power performance.
One approach to meeting these challenges is to improve chemical reactions at elevated temperatures. The other circumvents the difficulties by storing the magnesium cation in its complex forms. Neither approach is practical.
Yan Yao, Cullen professor of electrical and computer engineering at the University of Houston and corresponding co-author of the article, said the groundbreaking results came from the combination of an organic quinone cathode and a new solution of tailor-made boron cluster electrolyte.
“We have demonstrated heterogeneous enolization redox chemistry to create a cathode that is not hampered by the challenges of ionic dissociation and solid state diffusion that have prevented magnesium batteries from functioning effectively at room temperature,” Yao said. “This new class of redox chemistry bypasses the need for solid state intercalation while storing only magnesium, instead of its complex forms, creating a new paradigm in the design of magnesium battery electrodes.”
Yao, who is also a principal investigator at the Texas Center for Superconductivity at UH (TcSUH), is a leader in the development of multivalent metal ion batteries. His group recently published a review article in Energy of nature on the roadmap for better multivalent batteries.
TRINA researchers have made enormous progress in the field of magnesium batteries, including developing highly recognized and efficient electrolytes based on boron cluster anions. However, these electrolytes had limitations in supporting high battery cycle rates.
“We had clues that electrolytes based on these weakly coordinated anions could in principle have the potential to withstand very high cycling rates, so we worked on fine-tuning their properties,” said Rana Mohtadi, senior scientist in the department of TRINA materials research and corresponding co-author. “We tackled this problem by focusing our attention on the solvent in order to reduce its binding to magnesium ions and improve bulk transport kinetics.
“We were fascinated by the fact that the plated magnesium of the modified electrolyte remains smooth even at ultra-high cycling rates. We believe this reveals a new side of the electrochemistry of magnesium batteries.”
The work is in part a continuation of earlier efforts described in 2018 in Joule and involved several of the same researchers. In addition to Yao and Mohtadi, the co-authors include early authors Hui Dong, former member of Yao’s lab and now post-doctoral researcher at the University of Texas at Austin, and Oscar Tutusaus of TRINA; Yanliang Liang and Ye Zhang from UH and TcSUH; and Zachary Lebens-Higgins and Wanli Yang from Lawrence Berkeley National Laboratory. Lebens-Higgins is also affiliated with Binghamton University.
“The new battery is almost two orders of magnitude higher than the power density achieved by previous magnesium batteries,” Dong said. “The battery was able to continue operating for over 200 cycles with a capacity retention of about 82%, which shows high stability. We can further improve the cycle stability by tailoring the properties of the membrane with a capacity of improved intermediate trapping. “
Tutusaus said the work suggests the next steps towards high performance magnesium batteries.
“Our results define the direction of the development of cathode materials and high performance electrolytic solutions for magnesium batteries and uncover new possibilities of using metals with high energy density for rapid energy storage,” he said. he declares.
Lean electrolyte design is a game-changer for magnesium batteries
Hui Dong et al. High power Mg batteries activated by heterogeneous enolization redox chemistry and weakly coordinated electrolytes, Energy of nature (2020). DOI: 10.1038 / s41560-020-00734-0
Provided by the University of Houston
Quote: Findings highlight new possibilities for magnesium batteries (November 30, 2020) retrieved December 1, 2020 from https://techxplore.com/news/2020-11-discoveries-highlight-possabilities-magnesium-batteries.html
This document is subject to copyright. Other than fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.
[ad_2]
Source link