
[ad_1]
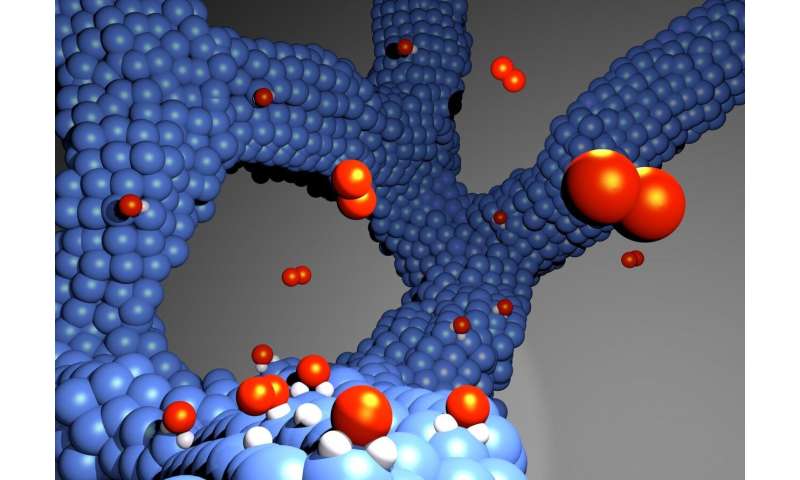
The new electrocatalyst for hydrogen fuel cells is made of a thin network of platinum-cobalt alloys and, unlike catalysts in common use today, does not require a carbon support. Credit: Gustav Sievers
About 1 billion cars and trucks zoom on the world’s roads. Only a few run on hydrogen. That could change after a breakthrough made by researchers at the University of Copenhagen. Discovery? A new catalyst that can be used to produce less expensive and much more durable hydrogen vehicles.
Hydrogen vehicles are a rare sight. Part of this is because they rely on a large amount of platinum to act as a catalyst in their fuel cells – around 50 grams. Typically, vehicles only need about five grams of this rare and valuable material. In fact, only 100 tonnes of platinum are extracted annually in South Africa.
Now, researchers in the chemistry department at the University of Copenhagen have developed a catalyst that does not require such a large amount of platinum.
“We have developed a catalyst which, in the laboratory, only needs a fraction of the amount of platinum that current hydrogen fuel cells for cars. We are approaching the same amount of platinum as needed for a conventional vehicle. At the same time, the new catalyst is much more stable than catalysts used in current hydrogen vehicles, ”explains Professor Matthias Arenz from the Chemistry Department.
A paradigm shift for hydrogen vehicles
Sustainable technologies are often challenged by the limited availability of the scarce materials that make them possible, which in turn limits scalability. Due to this current limitation, it is impossible to simply replace the world’s vehicles with hydrogen models overnight. As such, the new technology is a game changer.
“The new catalyst can allow hydrogen vehicles to be deployed on a much larger scale than would have ever been possible in the past,” says Prof. Jan Rossmeisl, head of the high entropy alloy catalysis center at the Department. of chemistry from UCPH.
The new catalyst dramatically improves fuel cells, making it possible to produce more power per gram of platinum. This makes the production of hydrogen fuel cell vehicles more sustainable.
More durable, less platinum
Because only the surface of a catalyst is active, as many platinum atoms as possible are needed to coat it. A catalyst must also be durable. This is where the conflict lies. To gain as much surface area as possible, today’s catalysts are based on platinum nanoparticles that are coated with carbon. Unfortunately, carbon makes catalysts unstable. The new catalyst is distinguished by its absence of carbon. Instead of nanoparticles, the researchers developed an array of nanowires characterized by surface abundance and high durability.
“With this breakthrough, the notion of hydrogen vehicles becoming mainstream has become more realistic. This allows them to become cheaper, more durable and more durable, ”says Jan Rossmeisl.
Dialogue with the automotive industry
The next step for the researchers is to increase their results so that the technology can be implemented in hydrogen vehicles.
“We are in discussions with the auto industry on how this breakthrough can be implemented in practice. Things therefore look very promising, ”says Professor Matthias Arenz.
The research results have just been published in Materials from nature, one of the leading scientific journals for materials research. This is the first article to which all researchers from the basic research center, “Center for High Entropy Alloy Catalysis (CHEAC)”, have contributed. The center is a so-called center of excellence, supported by the Danish National Research Foundation.
“At the center, we are developing new catalyst materials to create sustainable chemicals and fuels that help society make the chemical industry greener. The fact that it is now possible to increase the production of hydrogen vehicles, and in a sustainable manner, is a big step forward. », Says center head Jan Rossmeisl.
Platinum-free catalysts could make cheaper hydrogen fuel cells
Autonomous Pt – CoO networks combining a high specific activity and a large surface area for the reduction of oxygen, Materials from nature (2020). DOI: 10.1038 / s41563-020-0775-8, www.nature.com/articles/s41563-020-0775-8
Provided by the University of Copenhagen
Quote: Fuel Cells for Hydrogen Vehicles Last Longer (August 24, 2020) Retrieved August 24, 2020 from https://phys.org/news/2020-08-fuel-cells-hydrogen-vehicles-longer.html
This document is subject to copyright. Other than fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.
[ad_2]
Source link