
[ad_1]
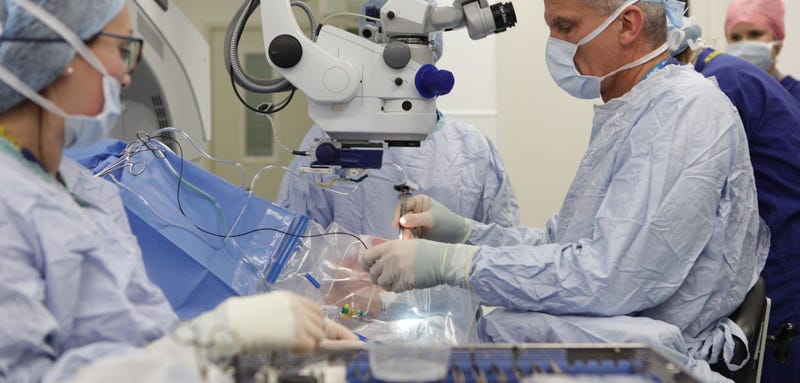
An 80-year-old UK woman is the first patient to undergo gene therapy to treat age-related macular degeneration, the most common cause of vision loss in the world.
It's too early to tell if the procedure worked, reports the BBC, but for the millions of people worldwide likely to develop age-related macular degeneration, or AMD, the experimental intervention has nevertheless marked an important medical turning point.
The patient, Janet Osborne from Oxford, has both eyes, but her left eye is more affected. The procedure to treat his AMD was performed by Robert MacLaren, an ophthalmologist at Oxford University, at John Radcliffe Hospital in Oxford, according to a university press release.
According to the University, Osborne wrestles with daily activities such as sewing, reading and recognizing faces due to poor vision caused by his condition. It has the dry type of AMD, also called atrophic AMD, which is more common and more difficult to treat than the wet, or neovascular, form of AMD. In dry form, the cells of the macula – the part of the retina responsible for central vision and development – gradually disappear and are not reconstituted. This causes visual deterioration in the form of haze, gaps or burrs when a person is looking directly at an object, while the peripheral vision remains intact.
AMD is a leading cause of vision loss worldwide and is the leading cause of vision loss and blindness in Americans over the age of 65, according to the US Center for Disease Control and Prevention. The CDC warns that between 48 and 88 million Americans could be affected by 2050.
According to the BBC, Osborne is the first of 10 patients treated in the FOCUS clinical trial, which aims to treat AMD through gene therapy. The trial is funded by NIHR's Oxford Biomedical Research Center and is sponsored by Gyroscope Therapeutics, a British company that develops gene therapies for various eye diseases.
"I did not think of myself. I was thinking of other people, "Osborne reportedly said after his surgery in the Oxford press release. "For me, I hope my eyesight does not get worse. It would be fantastic. This means that I would not be such a nuisance for my family. "
For the operation, Osborne received a local anesthetic. The doctors detached the retina from his left eye and injected him with a synthetic virus (Osborne's right eye was not treated because it was an experimental procedure). The virus contained a modified DNA sequence called retinal pigment epithelium (RPE) intended to repair the genetic defect responsible for AMD, a known inherited disease. A virus was used to deliver the DNA sequence, infecting the retinal cells.
Once the virus is at work in a retinal cell, it releases synthetic DNA and "the cell begins to make a protein that we believe can alter the disease, thereby correcting the imbalance of inflammation caused by the complement system, "MacLaren said in the press. Release. The complement system is a protein system that fights bacteria, but in macular degeneration, this part of the immune system becomes overactive and falsely attacks retinal cells. The goal of the new gene therapy is to stop the complement system, "but at a very specific point in the back of the eye, so that the patient is not affected by it and we hope that in the future this will slow the progression of macular degeneration, "said MacLaren.
As noted, it is still too early to say whether gene therapy has halted the deterioration of Osborne's left eye. It will be closely monitored in the coming weeks and months. The good news is that this gene therapy should only be performed once. It is important to note, however, that this intervention is not a restorative therapy – it is designed to stop, not reverse, degeneration caused by AMD. Ideally, this new therapy, assuming it works, would be used in patients in the early stages of AMD, before too much retinal damage occurs.
A 13-year-old boy is the first person in the United States to receive recently approved blindness gene therapy
On Tuesday, a 13-year-old boy from New Jersey was at the center of the history of medicine as he became the …
Read more
This is not the first gene therapy used to treat blindness. In March 2018, an FDA-approved gene therapy called Luxturna was used to treat a rare and hereditary form of blindness called Leber's congenital amaurosis.
[University of Oxford, BBC]
[ad_2]
Source link