[ad_1]
New research conducted by Harvard researcher Amy Wagers has shown that gene editing machines can be delivered directly to the stem cells where they live, rather than in a dish. Published in Cell Reports, the results have major implications for biotechnology research and the development of therapies for genetic diseases.
"If you want to modify a genome to correct a genetic mutation causing disease, you have to change it into the relevant stem cells," said Wagers, a professor of regenerative stem cell biology within the Forst family. "If you do not change the stem cells, all the cells that you repair may eventually be replaced by diseased cells fairly quickly. If you repair stem cells, they will create healthy cells that can eventually replace diseased cells. "
But securing stem cells is harder than it appears. As it stands, stem cells must be extracted, kept alive and in good health, genetically modified, and then reintroduced into the patient's body. The process disrupts cells, which can ultimately be rejected or not transplanted to the patient.
Each type of stem cell lives in its own "niche", well protected in hard-to-reach areas such as bone marrow. "When you extract stem cells from the body, you extract them from the very complex environment that feeds and supports them, and they are sort of shocked," said Wagers. "Isolating the cells changes them. Cell transplantation changes them. Making genetic changes without doing this would preserve the regulatory interactions of the cells – that's what we wanted to do. "
Virus transport
The Paris group has used an adeno-associated virus (AAV) that infects human (and mouse) cells – but does not cause disease – as a transport vehicle. Building on their earlier work on mice with Duchenne muscular dystrophy, Wagers and his colleagues have created various AAV packages to deliver a cargo of genetic modification to different types of stem cells, progenitors of the skin, blood and muscles.
"It was a real collaboration between specialized labs in several different bodies," said Jill Goldstein, a postdoctoral fellow at Wagers Lab and co-lead author of the study. "We set up experiments in our bodies of interest, analyzed them, compared notes and made adjustments in a kind of scientific assembly line. None of us could do it alone. It takes a lot of hands and the team approach has made the game really fun. "
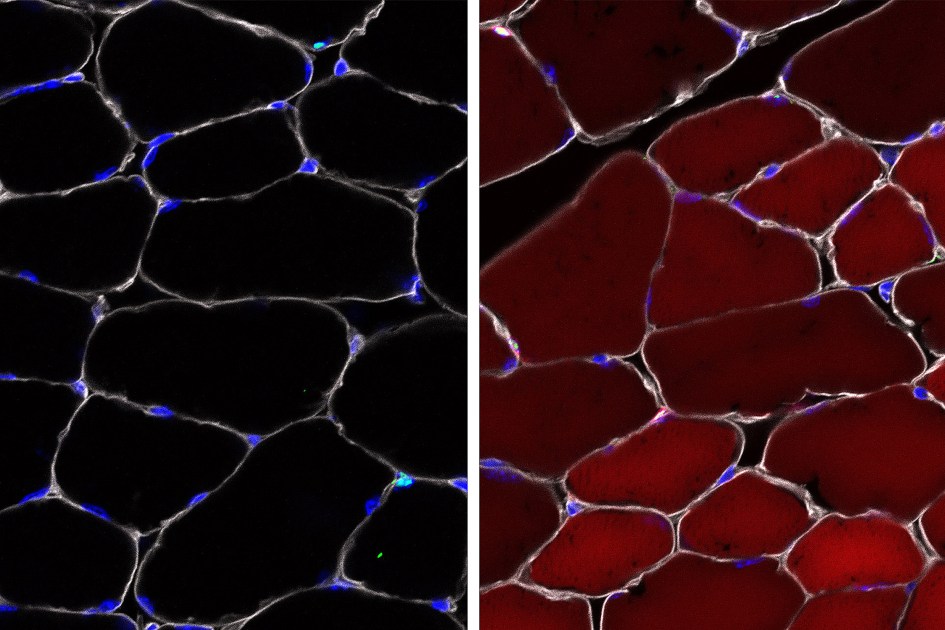
To test whether their AAV complexes were successful in delivering, the researchers used mice that act as reporter systems via a "reporter" gene that is normally silenced but that can be activated by gene editing. When the reporter gene is activated, the cell becomes bright red and fluorescent.
Up to 60% efficiency
The researchers observed that in skeletal muscle, up to 60% of stem cells became fluorescent red. In cells that give rise to different types of skin cells, up to 27% of cells have become red. Up to 38% of bone marrow stem cells (which make blood) have been modified. It may seem weak, but the blood turns so fast that in some cases even a single healthy stem cell can be enough to save a defect.
"Until now, the concept of healthy gene transfer to stem cells with the help of AAV has not been practical because these cells divide so rapidly in living systems – from so that the delivered genes will be diluted cells quickly, "said Sharif Tabebordbar, a former student of Harvard's Department of Regenerative Biology and Stem Cells and now a postdoctoral fellow at the Broad Institute. "Our study shows that we can permanently modify the genome of stem cells, and therefore of their progeny, in their normal anatomical niche. There is a lot of potential to advance this approach and develop more sustainable therapies for different forms of genetic diseases. This includes different forms of muscular dystrophy, where tissue regeneration is a very important factor. "
"We have examined the skin of these Wagers-transduced AAV-transduced mice and are delighted to see that many dermal cells have also been successfully modified," said Ya-Chieh Hsu, Alvin and Associate Professor Esta Star at the stem cell research and regeneration. Biology. "These cells included dermal adipocytes and cells that help regulate other stem cells in the skin. We have always needed a tool that allows us to rapidly manipulate dermal cells in vivo. It is therefore for us a dream come true. "
"Things could start going very fast"
The introduction of gene therapy directly into a living system is an obstacle for biotechnology companies seeking to develop therapies for diseases such as spinal muscular atrophy.
"It's a very important resource for the community for two reasons," said Wagers. "First, it changes the way we can study stem cells in the body. The AAV approach allows researchers to study the importance of different genes for stem cells in their original environment, much faster than ever before. Because the delivery system is very robust, it can also be used to target genes that affect many different tissues.
"Second, it's an important step in the development of effective gene therapies. The approach we have developed bypasses all the problems that you pose by extracting stem cells from a body and allows you to fix a genome permanently. AAVs are already used clinically for gene therapy, so things could begin to evolve very quickly in this area. "
This study was funded in part by grants and grants from Harvard University (Star Family Challenge Award, Dean's Initiatives Fund and Harvard Stem Cell Institute's Blood Program Pilot Project), New York Stem Cell Foundation, National Institutes of Health, Harvard Stem. The Cell Institute Junior Faculty Award, the Pew Charitable Trusts, the Odyssey Award from the Smith Family Foundation and the American Cancer Society, among others.
[ad_2]
Source link

