
[ad_1]

In this December 8, 2020 photo, U.S. President Donald Trump speaks during the Operation Warp Speed vaccine summit in the Eisenhower Executive Office building adjacent to the White House in Washington, DC. Operation Warp Speed (OWS) is a public-private partnership initiated by the United States government to facilitate and accelerate the development, manufacture and distribution of COVID-19 vaccines, therapeutics and diagnostics. Operation Warp Speed was first reported in the media on April 29, 2020 and was officially announced by the Trump administration on May 15, 2020. Credit: VOA
Vaccine development is usually measured in years, not months. But as the COVID-19[feminine la pandémie fait rage, les scientifiques se précipitent et battent des records pour développer une immunisation qui offre une protection contre le virus.
La communauté scientifique du pays est également confrontée à un autre obstacle: convaincre le public que le vaccin COVID-19 est sûr et à quel point il est important de se faire vacciner contre le COVID-19 en premier lieu.
«Même le vaccin le plus efficace ne peut pas nous protéger ou protéger nos proches si les gens ont peur de le prendre ou ne veulent pas le prendre», a déclaré Kathleen Mullane, directrice des essais cliniques sur les maladies infectieuses chez Université de Chicago Médicament. «Nous savons que les choses avancent plus vite que jamais, mais la communauté scientifique du pays a coopéré et collaboré comme jamais auparavant et nous sommes absolument déterminés à nous assurer que tout ce qui est finalement approuvé fonctionne et est sûr. Je vais me faire vacciner et je recommande la vaccination à ma famille et mes amis car je crois en l’innocuité et l’efficacité de ces agents.
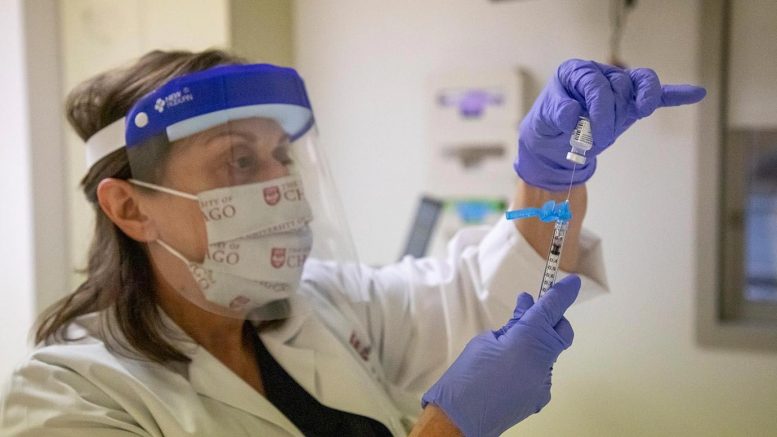
Un professionnel de la santé se prépare à administrer le vaccin COVID-19 à l’Université de médecine de Chicago. Crédit: Photo de Jordan Porter-Woodruff
Les progrès rapides sur un vaccin COVID-19 signifie que les données concernant la sécurité et la durabilité à long terme de ces vaccins continueront de circuler longtemps après qu’un vaccin a été approuvé pour une utilisation d’urgence. Néanmoins, ceux qui s’interrogent sur la sécurité des vaccins peuvent être encouragés par le fait qu’en dépit de la rapidité avec laquelle ces vaccins ont été développés, les importants points de contrôle réglementaires et d’évaluation conçus pour protéger les patients ont été suivis. Ces jalons aident à déterminer dans quelle mesure un vaccin sera sûr et efficace et si les avantages valent ou non des risques potentiels.
Opération Warp Speed
Avant la pandémie du COVID-19, faire passer un nouveau vaccin du concept à l’approbation pouvait prendre 10 ans et des milliards de dollars. Avec seulement un candidat vaccin sur 10 sur le marché, le développement de vaccins est une proposition risquée pour les fabricants de produits pharmaceutiques.
Pour ceux qui ne sont pas familiers avec le processus méthodique de la recherche clinique, le processus peut sembler extrêmement lent. Premièrement, les chercheurs doivent étudier la structure et le comportement infectieux d’un pathogène. Ensuite, ils découvrent comment amener le corps humain à produire au mieux une réponse immunitaire pour lutter contre elle. Ensuite, l’innocuité et l’efficacité du vaccin sont testées – d’abord à l’aide de modèles cellulaires, animaux et mathématiques, puis dans des essais cliniques humains impliquant des milliers de participants. Ce n’est qu’alors que le processus d’approbation fédérale pourra commencer.
Des dizaines de vaccins contre la SRAS-CoV-2 virus sont en cours de développement par des sociétés pharmaceutiques mondiales, mais à ce jour, seuls quelques-uns ont atteint des essais cliniques de phase 3 à grande échelle. Dans les essais de phase 3, des dizaines de milliers de volontaires participent pour tester la sécurité et l’efficacité de la vaccination. Jusqu’à présent, 11 essais de phase 3 ont été lancés dans le monde, bien que d’autres soient attendus dans les mois et les années à venir alors que d’autres efforts de recherche progressent dans le pipeline.
Ils bénéficient d’un coup de pouce grâce à l’opération Warp Speed, une collaboration entre l’industrie pharmaceutique et le gouvernement fédéral. Pour compenser le coût du développement du vaccin COVID-19 et aider à mobiliser le plus rapidement possible les vaccins approuvés auprès du public américain, le gouvernement a mis en place près de 10 milliards de dollars de fonds fédéraux. Cela a considérablement accéléré le calendrier du développement des vaccins grâce aux essais cliniques, à l’examen de la FDA et à la distribution de masse d’un vaccin.
Tous ces facteurs signifient à leur tour qu’une fois qu’un vaccin a franchi les étapes critiques d’innocuité et d’efficacité et a reçu l’approbation pour une utilisation d’urgence du gouvernement fédéral, les organisations de soins de santé ont pu commencer à fournir le vaccin aux patients en quelques jours. Par exemple, le vaccin à ARNm Pfizer / BioNTech a été approuvé pour une utilisation d’urgence par la FDA le 10 décembre 2020; les travailleurs de la santé étaient vaccinés le 14 décembre.
What are the different types of vaccines? Credit: Video courtesy of UChicago Medicine
It should also be reassuring to note that almost 200 years of vaccine development have generated a number of highly effective and safe vaccine platforms, requiring less time and effort to produce new types of vaccines. Recycling existing vaccine technology allows researchers to spend their time identifying the best targets that will produce the strongest immune response with the fewest side effects.
“In fact, most of the work on developing the vaccine platform is already done,” said Habibul Ahsan, director of the Institute for Population and Precision Health at the University of Chicago Medicine. “You just have to do the remaining part, which is to add the right viral antigens to the already proven platform and make sure it is safe and effective in humans. Even in the last five to ten years alone, we have made great strides in developing new types of vaccine platforms like the ones being tested for SARS-CoV-2. “
Vaccines work by presenting the body’s immune system with certain virus proteins called antigens, which activate the immune response to generate antibodies that protect against the disease.
The vaccine candidates that are currently making headlines use mRNA and vector platforms. Vector-based vaccines have been developed in the past for diseases such as SARS, MERS, and more particularly the deadly Ebola virus; and mRNA vaccines have already been tested to prevent Zika virus.
These next-generation immunizations have never been tried on such a large scale before, but there is already evidence that these platforms are safe and effective, with a reduced risk of side effects generated by previous types of vaccines such as than live attenuated or deactivated vaccines. – antivirus vaccines.
“The mRNA and vector vaccines are a newer technology; the first products were developed in 1999, ”said Mullane. “Based on our understanding of human biology, there is no reason to believe that they should pose a greater risk than any of the more traditional types of vaccines. On the contrary, the biggest concern is the duration of their effectiveness. Preliminary efficacy data to date is extremely promising. “
How do vaccines work? Video courtesy of UChicago Medicine
Combine clinical phases
To speed up development, numerous COVID-19 vaccine trials are being conducted in studies combining phases 1, 2 and / or 3 where researchers start by vaccinating a smaller number of healthy volunteers. As the trial continues, if the vaccine seems safe, then it opens up to more participants, such as those with pre-existing health conditions. Large-scale phase 3 efficacy trials ultimately include tens of thousands of volunteers. The current range of trials includes a variety of vaccine types – both proven models and next-generation approaches.
Before a vaccine can receive federal approval, even for emergency use, investigators must wait until tens of thousands of volunteers receive their experimental vaccine. Then they wait long enough for some of those volunteers to be exposed to COVID-19, which indicates the effectiveness of each vaccine. Scientists are also studying whether those who received the vaccine – compared to a placebo – had less severe forms of the disease. Without data conclusively demonstrating that vaccines are both safe and effective, they are not approved for use by the general public.
Once a clinical trial collects enough data to show that a vaccine is both safe and effective, the drug company submits the data to the FDA, where it is reviewed and reanalysed by federal statisticians and an external advisory board. scientific and medical experts. Since most COVID-19 vaccines will be submitted to the FDA before long-term data entry, it is likely that a vaccine will receive emergency use authorization rather than full approval. This was the case with the Pfizer / BioNTech mRNA vaccine, which was approved for emergency use by the FDA on December 10, 2020, and the Moderna mRNA vaccine, eight days later.
The speed at which these trials progress has raised the question of whether or not they are safe. Scientists are aware of the lingering mistrust due to the politicization of the pandemic and the historic medical deprivation of some communities; they are therefore careful not to overestimate the initial results of ongoing clinical trials and to be transparent about the risks involved.
How does the FDA approve vaccines? Video courtesy of UChicago Medicine
Many are making efforts to go out into the community to speak with potential volunteers, address their concerns, and offer the opportunity to participate, especially in large-scale Phase 3 trials. For example, at UChicago Medicine, clinicians take mobile medical units to surrounding neighborhoods to bring the vaccine directly to people’s doors.
“Many recruiting centers for these trials are not easily accessible to minority and low-income populations,” Ahsan said. “But UChicago Medicine has worked hard to build strong relationships with our local community and make sure they know we are there to serve them.
What risks do vaccines pose?
Like most medical treatments, any vaccine comes with some degree of risk. Side effects are usually mild, ranging from pain at the injection site to mild fever and body aches. In one in 100,000, vaccines can cause severe allergic reactions. Even rarer (the estimate is one in a million) is an increased risk of developing autoimmune conditions that affect the nervous system, such as Guillain-Barre syndrome.
Two separate studies of live non-replicating vector virus vaccines – the UK-based AstraZeneca Phase 3 vaccine trial and the US Janssen Phase 3 vaccine trial – were briefly suspended after one participant underwent an unexplained medical event called an “adverse reaction” which may have been linked to their participation in the study. The two have since resumed after researchers and regulators determined there was no clear link between the vaccine and medical events and found them safe enough to continue. No adverse events have yet been linked to mRNA vaccine candidates, except for a handful of allergic reactions requiring EpiPens.
To protect against uncertainty, the FDA added additional rules to ensure increased safety by specifying checkpoints for accelerated testing of COVID-19. This includes the requirement for researchers to collect at least two months of follow-up data from the majority of participants in each trial, even though early data shows promising results, and long-term safety and efficacy of up to two years after receiving the vaccines.
Return to normal
Next comes the challenge of manufacturing and distributing a vaccine. Full deployment can take months to get enough bundles for the general public; In the meantime, authorities will prioritize distribution to those most at risk of contracting COVID-19 or those most likely to suffer the most severe effects of the disease, such as healthcare workers, the elderly, adults with pre-existing illnesses and essential workers.
As a new vaccine is distributed, clinical trials will continue and data will continue to flow on its long-term efficacy and possible safety concerns. This will allow researchers and healthcare providers to adapt the distribution if necessary.
Realistically, the general public is unlikely to have access to a vaccine until this summer. This is much later than Operation Warp Speed’s initial goal of having 300 million doses available by January, but much faster than any other vaccine development effort to date.
Those interested in participating in ongoing COVID-19 vaccine trials – including potential new vaccine candidates in future trials – can register for the UChicago Medicine COVID-19 Vaccine Registry at covidvaccinestudies.uchicago.edu.
[ad_2]
Source link