
[ad_1]
Could the poop of some cancer patients be the key to treating certain cancers in all patients?
What does cancer have to do with poo? In recent years, researchers around the world, including ourselves, have realized that intestinal bacteria – what we call the gut microbiome – cancer patients may be the key to improving therapies anticancer drugs for patients. It is not clear how this is going, but it may be related to the ability of intestinal bacteria to stimulate our natural immune responses.

Michael Conroy / AP Photo
The intestinal microbiome includes all microorganisms of the gastrointestinal tract. But recent research suggests that microbes in the gut might not be idle onlookers. On the contrary, they can be critical in helping patients respond to new drugs called "immune checkpoint inhibitors" that help immune cells recognize and attack tumor cells. My goal as an oncologist specializing in melanoma is to develop new approaches to treat advanced cancer, particularly in patients whose cancers do not respond to these otherwise powerful immunotherapies. To that end, we decided to examine whether certain types of microbes could enhance the efficacy of immune checkpoint inhibitors and designed a single clinical trial to determine whether the intestinal microbiome influences efficacy. of these drugs in patients with melanoma.
The Intestinal Microbiome and Cancer Immunotherapy
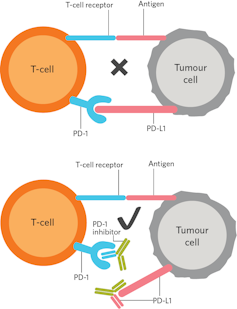
The medical community has long known that if the immune system is able to "see" the cancer cells, it often does not happen to them destroy. This occurs because cancers can "hide" immune cells, thus avoiding the natural anti-cancer responses usually generated by the immune system. Cancers overproduce proteins such as PD-L1 that they present on their surface to evade immune surveillance. The cancer cells use the PD-L1 protein to essentially sleep the immune cells.
UPMC CC BY-NC
Inhibitors PD-1 and PD-L1 block the interaction between PD-L1 on the cancer cell and the PD-1 receptor on immune cells from the body. This allows the immune system to recognize and kill these cancer cells.
These results are the result of decades of painstaking work by scientists around the world, including Tasuku Honjo in Japan and James Allison with Arlene Sharpe and Gordon Freeman in the United States. Their work has allowed scientists to develop drugs such as PD-1 and CTLA-4 inhibitors that help the body's natural immune system recognize cancer as a foreign entity and, in doing so, trigger a wave of T cells that recognize and kill cancer cells.
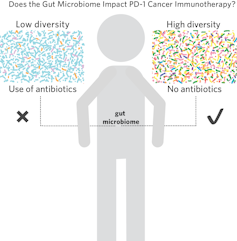
These revolutionary agents transformed some advanced cancers into a chronic disease; in melanoma, PD-1 inhibitors produce lasting responses in 30-40% of patients. However, these medications do not work in other 60-70% of melanoma patients for a multitude of reasons, including not having the right microbes in the intestine – a condition called "intestinal dysbiosis." [19659002] How exactly does bacteria in its gut affect the immune system? It turns out that it's complicated.The "intestinal microbiome" includes about 100 trillion microbial cells that outnumber all human cells in the body. body and form an ecosystem that influences host physiology, nutrition, metabolism and immune function.The work of several prominent groups like Gustave Roussy, the University of Chicago, the MD Anderson Cancer Center and The University of Texas at Dallas suggest that not having the right bugs explains why PD-1 inhibitors do not work in some patients.
The Microbiome intestinal and cancer immunotherapy
By Kateryna Kon / shutterstock.com
Bacteria are hard work. In all published studies, researchers obtained stool samples from cancer patients before and after PD-1 immunotherapy. They sequenced the genomes of the bacteria to determine their identity. Then, they used various calculation techniques to calculate the exact species in each of the different stool samples, and to determine the relative diversity and abundance of bacterial species between responders (whose tumors decreased) and patients no answering machines. Several studies have explored the effect of fecal administration in human responder patients to tumor-bearing mice and have shown that mouse tumors regressed – suggesting that some elements in crap facilitated treatment cancer.
When we saw these data, we wondered if the administration of microbes derived from responders – via a fecal transplant – could reduce tumors by correcting the balance of microbes in the intestine just like in tumor-bearing mice. We were particularly curious about whether the approach could be used to treat patients who do not respond to anti-PD-1 immunotherapy. Although the various groups, including ours, have studied stool samples from cancer patients, the bacterial species badociated with responder patients varied from one study to the next. This could be due to technical differences in the way these samples were collected and badyzed; but it also highlights the difficulties inherent in badyzing the responsibility of a single bacterial species or group of bacteria in mediating these effects.
But determining the exact bacterium (or group of bacteria) responsible for this effect could take a long time. There are between 10 and 100 trillion bacteria in the human gut – considerably more than the US debt ceiling starting in 2018. Experimenting with one species at a time could take decades. Instead, we decided to use "bacterial badtails" derived from the fecal matter of cancer patients who had done an extraordinarily good treatment on PD-1.
This approach borrows ideas from the microbial world, particularly the importance of function on identity: which means that what a particular microbe, or collection of microbes, does is more important than his identity.
First trial in humans
In our first human trial, we collect the intestinal microbiome in patients whose cancers responded extraordinarily well after anti-PD immunotherapy. 1. By using this, we generate a fecal microbiome transplant – "FMT", or simply "a shit transplant."

Steven Senne / AP Photo
We select patients whose cancers have not been treated. not responded to anti-PD. -1 immunotherapy. Following a biopsy of their tumor, the patients then receive this "poop transplant" by colonoscopy with a PD-1 inhibitor drug called pembrolizumab. Fecal transplantation is followed by several pembrolizumab treatments after which the patient's response is evaluated. Responding patients continue to receive the drug to complete two years of therapy.
The links between the bacteria that we isolated and certain food elements led us to also monitor dietary intakes in this study and in patients receiving immunotherapy.
UPMC / Diwakar Davar CC BY-NC
Fecal transplants are highly effective in the treatment of life-threatening infections Clostridium difficile . Research suggesting that intestinal dysbiosis could be at the origin of other diseases has spawned a host of fecal transplant studies in other diseases ranging from inflammatory bowel disease to obesity and graft-versus-host disease, and even in autistic people with gastrointestinal disorders.
However, aside from our study, there is only one other study at Sheba Medical Center in Israel that tests this approach for treating advanced cancer.
We do not know if the manipulation of the microbiome can treat advanced melanoma. However, we hope that our research efforts will advance the science behind the microbiome in cancer patients receiving immunotherapy and uncover new links between cancer, the response to cancer immunotherapy and the gut microbiome, including factors that could affect it. Your poop can be critical to this effort – so think about giving us a sample.
Source link