[ad_1]
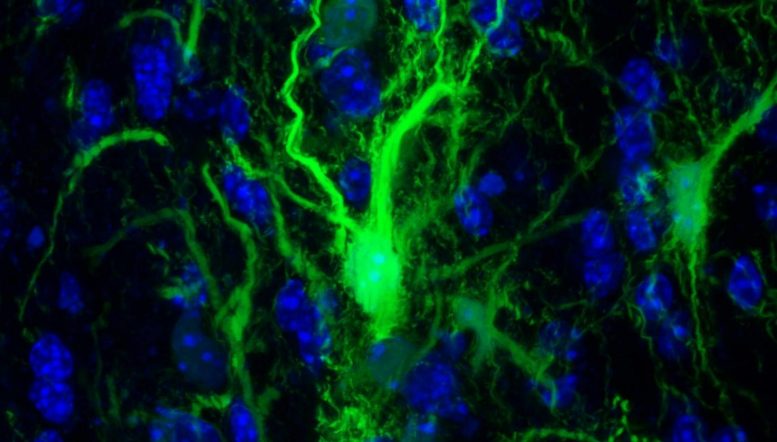
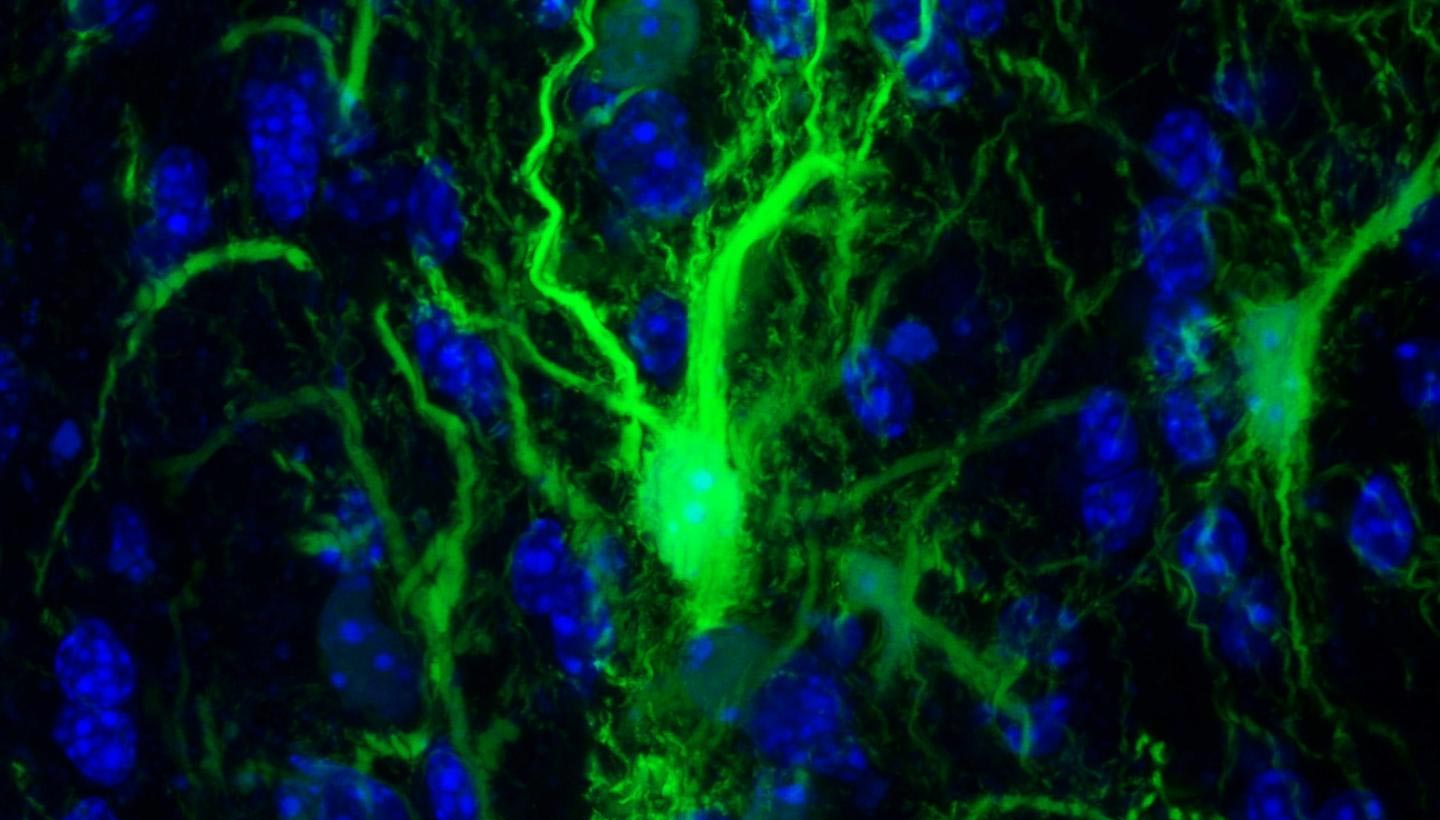
GRP confocol in the brain. Credit: Johns Hopkins Medicine
The transplanted brain stem cells survive without anti-rejection drugs in mice. By exploiting a characteristic of the immune system, researchers are opening the door to stem cell transplantation to repair the brain.
The ability to successfully transplant healthy cells into the brain without the use of conventional anti-rejection drugs could advance the search for therapies that help children born with a rare but devastating class of genetic diseases, including myelin, the protective coating that helps them send messages, does not form normally. About one in 100,000 children born in the United States will have one of these diseases, such as Pelizaeus-Merzbacher disease. This disorder is characterized by the absence in children of stages of development such as sitting and walking, involuntary muscle spasms and partial paralysis of arms and legs, all caused by a genetic mutation of myelin-forming genes.
"Because these conditions are caused by a mutation that causes dysfunction in a cell type, they are a good target for cell therapies, which involve transplanting healthy cells or cells that are designed to not have the condition to support. sick, damaged or missing cells. Says Piotr Walczak, MD, Ph.D., associate professor of radiology and radiology at the Johns Hopkins University School of Medicine.
The immune system of mammals is a major obstacle to our ability to replace these defective cells. The immune system works by quickly identifying "self" or "non-self" tissue and multiplying attacks to destroy non-self or "alien" invaders. Although beneficial in targeting bacteria or viruses, it is a major barrier to transplanted organs, tissues or cells, which must also be destroyed. Traditional anti-rejection drugs that, in general and in a non-targeted way, weaken the immune system often help to prevent tissue rejection, while leaving patients vulnerable to infection and other side effects. Patients must stay on these drugs indefinitely.
In an attempt to stop the immune response without causing side effects, the Johns Hopkins Medicine team looked for ways to manipulate T cells, the elite infection-fighting force of the system, which attacks foreign invaders .
In particular, Walczak and his team focused on the series of "co-stimulatory signals" that T cells must encounter in order to launch an attack.
"These signals are in place to help ensure that these immune system cells do not become unwanted and attack the body's own healthy tissues," said Gerald Brandacher, MD, professor of plastic and reconstructive surgery and scientific director of the research laboratory. on the allotransplantation of vascularized composites at the Johns Hopkins University School of Medicine and co-author of this study.
The idea, he says, was to exploit the natural tendencies of these co-stimulatory signals to form the immune system to the definitive acceptance of grafted cells as "self."
To do this, researchers used two antibodies, CTLA4-Ig and anti-CD154, that prevent T cells from triggering an attack when they encounter foreign particles by binding to the T-cell surface, essentially blocking the signal. to go". This combination has already been used successfully to block the rejection of solid organ transplants in animals, but has not yet been tested for cell transplantation to repair myelin in the brain, says Walczak. .
In a set of key experiments, Walczak and his team injected into the mouse brain the protective glial cells that produce the myelin sheath surrounding the neurons. These specific cells have been genetically modified so that researchers can monitor them.
The researchers then transplanted the glial cells into three types of mice: mice genetically engineered to not form the glial cells that create the myelin sheath, normal mice, and mice that were designed to be unable to develop an immune response.
Next, the researchers used the antibodies to block an immune response, stopping treatment after six days.
Every day, researchers used a specialized camera capable of detecting glowing cells and capturing images of the mouse brain, looking for the presence or relative absence of transplanted glia. Cells transplanted into control mice that did not receive the antibody treatment immediately began to die and the glow was no longer detected by the camera on the 21st day.
The mice that received the antibody treatment maintained significant levels of glial cells transplanted for more than 203 days, showing that they had not been killed by mouse T cells, even in those cases. 39, no treatment.
"The fact that there is no longer any glow showed us that the cells had survived the transplant, even long after stopping treatment," says Shen Li, MD, lead author of the study. study. "We interpret this result as a success in selectively inhibiting the T cells of the immune system from killing the transplanted cells."
The next step was to determine whether the transplanted glia had survived enough to do what glial cells normally do in the brain: create the myelin sheath. To do this, the researchers looked for key structural differences between the brains of mice with flourishing glia and those that did not, using MRI images. In the images, the researchers found that the cells of the treated animals actually populated the appropriate parts of the brain.
Their findings confirmed that transplanted cells were able to grow and assume their normal function of protecting neurons in the brain.
Walczak warned that these results are preliminary. They were able to release these cells and allow them to grow in a localized part of the mouse brain.
In the future, they hope to associate their findings with studies on methods of delivering cells to the brain to help repair the brain more holistically.
###
Other researchers participating in this study include Byoung Chol Oh, Chengyan Chu, Antje Arnold, Anna Jablonska, Georg Furtmuller, Huamin Qin and Miroslaw Janowski of Johns Hopkins University; Shen Li from Dalian City Central Hospital and Johns Hopkins University; Johannes Boltze of the University of Warwick; and Tim Magnus and Peter Ludewig of the University of Hamburg.
Reference: "Induction of immunological tolerance to myelin-restricted glenoid progenitor allografts" by Sheng Li, Byoung Chol Oh, Chengyan Chu, Antje Arnold, Anna Jablonska, Georg J Furtmiller, Hua-Min Qin, Johannes Boltze, Tim Magnus, Peter Ludewig , Mirosław Janowski, Gerald Brandacher and Piotr Walczak, September 16, 2019, Brain.
DOI: 10.1093 / brain / awz275
[ad_2]
Source link