[ad_1]
Spend a few minutes with Anthony Brasich and it quickly becomes clear that he is a special type. Pleasant. Friendly. Great sense of humor, with a penchant for classic one-liners.
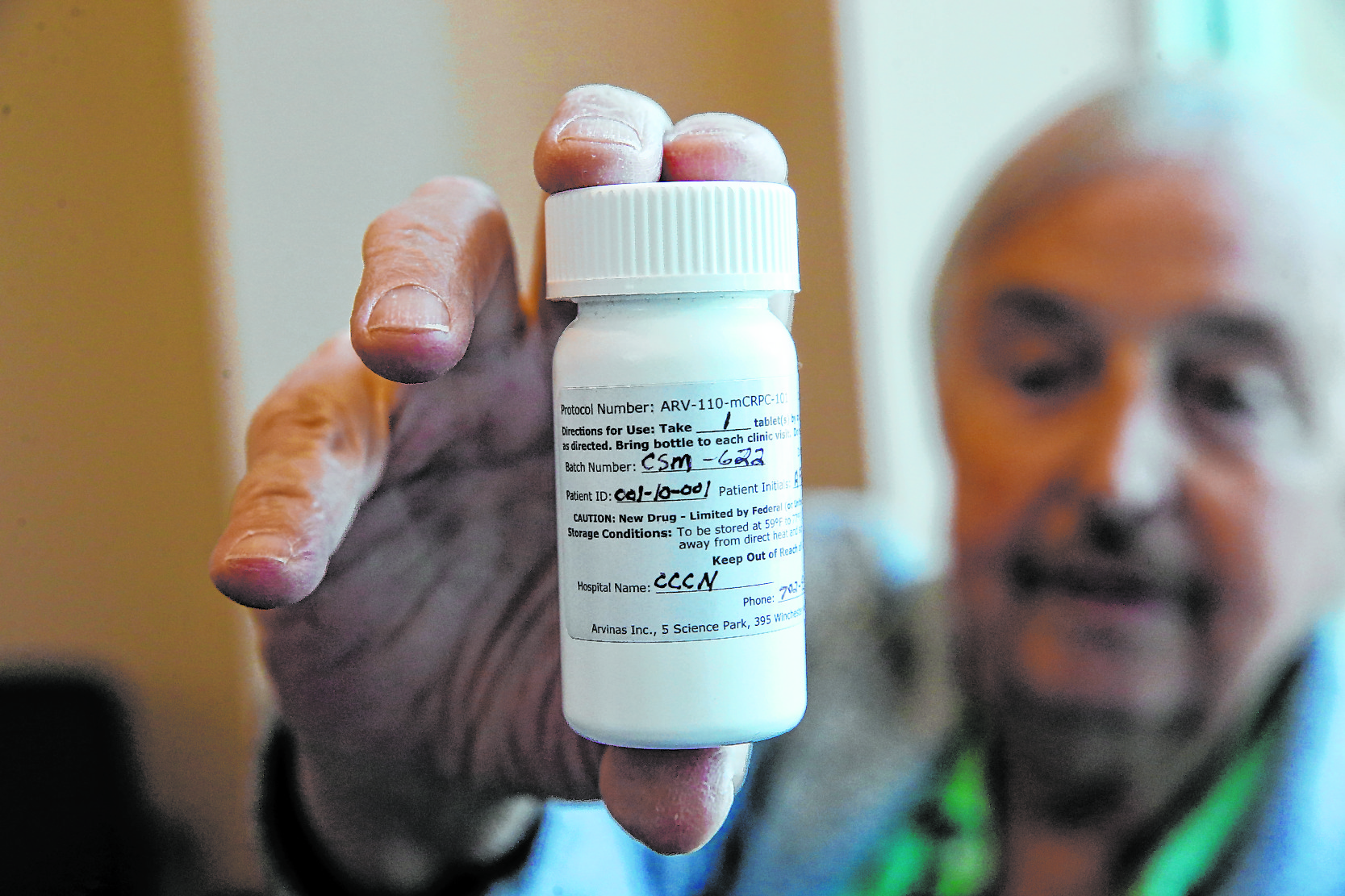
But if you need evidence that Brasich, from Las Vegas, really has a specificity, just look at the strange label on the pill bottle that contains his last drug once a day, which designates him as patient 001.
"It's me," said Brasich, laughing. "Hope that there will be another million."
Brasich, 71, was diagnosed about 10 years ago with metastatic prostate cancer at stage 4 and had only a few months to live. This singular designation on the pill bottle indicates that he is the first patient in the world to participate in Phase 1 clinical trials of an experimental drug called ARV-110.
Brasich, a retired casino dealer, has undergone several cancer treatments over the last decade. Many of them worked for a while and then did not work.
Two and a half months ago, Brasich began taking the ARV-110 in the form of a daily pill. He tolerates it well, feels reduced pain and even learned Thursday during a checkup that his PSA rate had stabilized. (High PSA or prostate specific antigen may indicate cancer.)
"I feel so much better than a few months ago," says Brasich. "With the other drug, I felt pain in the side and in the head, I could not walk and my vision was blurry, and now I'm fine."
Brasich's doctor, Dr. Nicholas Vogelzang, head of medical oncology at Nevada's comprehensive cancer centers, says that prostate cancer needs androgens, male hormones, to grow.
The ARV-110 destroys what is called the androgen receptor, which slows the progression of cancer.
Brasich has undergone chemotherapy, radiation therapy and other pharmacotherapies, as well as several experimental treatments. When Vogelzang told him about the ARV-110 clinical trial, he replied, "If you want to try, it has never been used in humans." I said, "Will I be considered a human ? "
"I think that a rat named Cecil was the first to use it, and now to me," he jokes.
In fact, Brasich said: "I was very anxious to do it (because) I'm in a position where everything was failing."
If all goes well, clinical trials on ARV-110 will continue with the gradual addition of more prostate cancer patients. The study will then move to the review phase of the drug's efficacy and comparison with existing prostate cancer treatments.
Even under ideal circumstances, the approval of the drug as a treatment option could be achieved within two to four years, Vogelzang said. But he is satisfied with what he has seen so far in Brasich.
A drop in the PSA rate of Brasich is "already remarkable", he says, "so we are very encouraged by these first patients"
Brasich is grateful that the drug seems to be helping him so far, and says it would be great if his participation in the study would cure his cancer.
But, he says, "more than anything, I think, it's helping others."
"I do not think so, like," OK, it'll work so I'm fine. "Well, if it works like that, but I'd rather see something in the future come out of it all.
"I think of the way I am first," he says, with tears in his eyes.
Contact John Przybys at [email protected] or at 702-383-0280. To follow @JJPrzybys on Twitter.
[ad_2]
Source link