[ad_1]
The Food and Drug Administration is expected to make a decision on using cabotegravir as PrEP by early 2022. Gilead’s semi-annual injectable and a monthly Merck pill called islatravir could also get the green light by 2025.
Cost could be a barrier
Federal agencies recently informed health insurers that almost everyone is now required to cover daily PrEP at no cost, including for medications, clinic visits and associated lab tests.
But these measures should not apply by default to any new approved form of PrEP, including cabotegravir. So insurers could potentially charge a substantial co-payment or other fee for this injectable drug – provided payers cover a drug that will likely have a list price much higher than the $ 30 per month that generic Truvada costs. the cheapest.
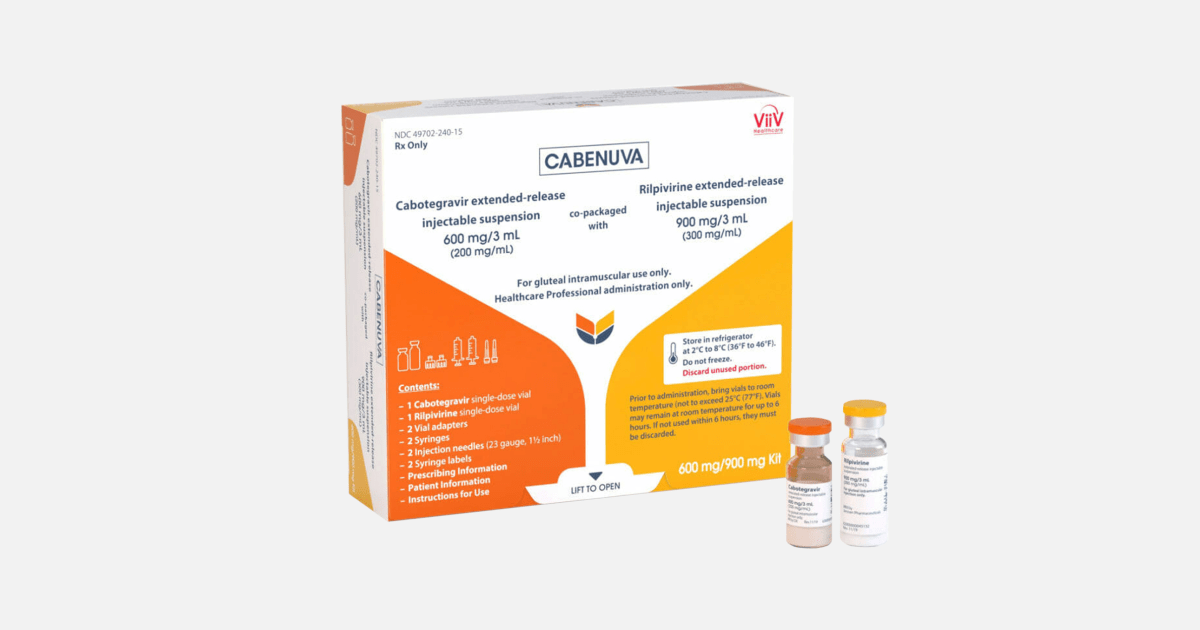
ViiV’s monthly injectable HIV treatment, Cabenuva, which includes cabotegravir plus the drug rilpivirine, costs $ 3,960 per injection, while the brand name Truvada costs $ 1,840 and Descovy costs $ 1,930 per month.
The US Task Force on Prevention Services is expected to recommend cabotegravir, as it did for daily PrEP two years ago, as an intervention with an evidence-based net benefit to ultimately eliminate the reimbursable costs for cabotegravir. This process could take years.
Two large surveys of gay and bisexual men presented last week at the 11th International AIDS Society HIV Science Conference revealed substantial interest in long-acting PrEP, but also concerns about the associated potential financial burden, particularly among men of color and those who reported riskier sexual behavior. In addition, many men have expressed a preference for newer HIV prevention drugs that require dosing even less frequently than every other month. These men’s wish could very well be granted – in time.
Give patients more choice
According to Brad Hare, clinical director of HIV for Kaiser Permanente in San Francisco, “the next generation of PrEP will be focused on individualization,” in which multiple forms of drug-based HIV prevention provide people at risk with “the options”. that work best for them at some point in their life.
Sharon Hillier, a prominent HIV researcher at the University of Pittsburgh, presented the interim results at last week’s conference of a mid-term PrEP study on islatravir. After the participants took six monthly doses of the aspirin-sized pill, they still had concentrations of the drug in their blood that should protect against the virus even eight weeks later.
Merck recently began recruiting participants for a pair of major trials that will compare the effectiveness of islatravir as a monthly form of PrEP to daily doses of Truvada or Descovy. Gilead is launching its own pair of large-scale efficacy trials for lenacapavir as an HIV prevention, following promising research in monkeys. The results of the two pairs of trials are expected in 2024.
In March, Merck announced promising results from a first human safety trial of an islatravir-infused subcutaneous implant and plans to begin a larger mid-term study of the implant. as protection against HIV for up to 12 months.
Participants at the recent conference were also told about the early development at the University of North Carolina of a removable, biodegradable implant that delivered antiretroviral drugs to mice for up to 180 days.
In addition, the HIV Vaccine Trials Network is developing cocktails of antibody treatments that the researchers hope will eventually prove effective as a prophylaxis against HIV.
Howard Brown’s Green said adding these countless prevention options to his arsenal could help him in his determined efforts to put his patients in the driver’s seat when it comes to protecting themselves from HIV.
“When a patient is a leader in their choice, in their medical decision-making, the results are always better,” she said.
“I always say the patient is Batman. We are Robin.
[ad_2]
Source link