
[ad_1]
Israel is in the final stages of negotiations with British pharmaceutical giant AstraZeneca to buy “millions” of doses of its vaccine, which is currently being tested, Prime Minister Benjamin Netanyahu said on Friday.
Netanyahu said that in the next few hours, “the final details will be worked out before an agreement is signed to purchase millions of doses of the coronavirus vaccine developed by AstraZeneca and the University of Oxford.”
This would be the third agreement signed by Israel to receive vaccinations following similar agreements with Moderna and Pfizer. Israel is also in talks with Russia to receive its “Sputnik V” vaccine.
Receive the daily edition of The Times of Israel by email and never miss our best articles Sign up for free
“My policy is that anyone who wants a vaccine should be able to get one,” Netanyahu said, alluding to recent government denials that he intended to make the vaccine mandatory.
“We will continue to work to make sure that we get as many vaccines for as many citizens, from as many sources and as quickly as possible,” he said.
Channel 13 reported that the deal would bring up to 10 million doses, which would immunize 5 million people.
Pfizer said Friday it was asking U.S. regulators to allow emergency use of its COVID-19 vaccine, starting the clock on a process that could bring limited first vaccines as early as next month.
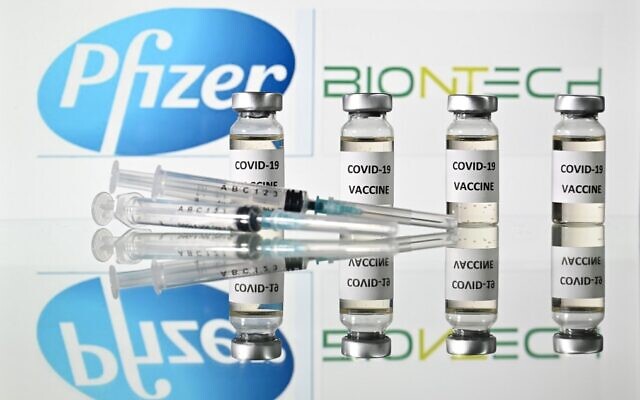
Illustration: Vials with attached COVID-19 vaccine stickers and syringes with the logo of the US pharmaceutical company Pfizer and its German partner BioNTech, November 17, 2020 (Justin Tallis / AFP)
Moderna is also expected to apply for emergency authorization within a few weeks.
AstraZeneca is not as far along in the process, but the company announced Thursday that in initial trials its vaccine was shown to safely produce a robust immune response in healthy elderly people.
The vaccine produced fewer side effects in people aged 56 and older than in younger people – a significant finding given that COVID-19 disproportionately causes serious illness in the elderly, the said. company by publishing the results of its Phase 2 trial.
The manufacturers said the vaccine is in larger and more comprehensive Phase 3 trials to confirm the results.
Immune responses to vaccines tend to decrease as people get older because the immune system gradually slows down with age.
This makes the elderly more vulnerable to infection from various diseases.
“As a result, it is crucial that COVID-19 vaccines are tested in this group which is also a priority group for vaccination,” Andre Pollard, professor at Oxford and lead author of the study results, published in The Lancet.
The phase 2 trial saw 560 participants, 240 of whom were over 70, divided into groups who received one or two doses of the vaccine, or a placebo.
Their immune responses were assessed on the day of vaccination and then repeatedly over the next several weeks.
The responses were “similar” across all age groups, the researchers said.
“Research shows that an immune response was generated in all age groups, including the cohort of participants over the age of 70,” said Michael Head, senior researcher in global health at the University of Southampton, who did not participate in the test. .
“Given that the elderly will be one of the priority groups to receive a vaccine when it becomes available, this is good news.”
The Oxford / AstraZeneca vaccine is one of 48 vaccines in human trials against COVID-19, according to the World Health Organization.
This month, Pfizer / BioNTech and Moderna announced the results of Phase 3 trials – which are much larger than Phase 2 and normally include tens of thousands of subjects – which suggested that both vaccine candidates were effective in preventing COVID-19.
Netanyahu spoke with Russian President Vladimir Putin on Monday about the possibility of purchasing the Russian coronavirus vaccine.
The Kremlin issued a statement saying Putin and Netanyahu discussed potential cooperation regarding the Russian vaccine, including its supply to Israel and even its production in the Jewish state.
Russia’s Sputnik V coronavirus vaccine has so far been shown to be 92 percent effective, the country’s sovereign wealth fund, which supports the program, said last week.

A vial of the experimental Russian coronavirus vaccine Sputnik V, in Moscow, Russia, September 15, 2020 (Alexander Zemlianichenko Jr / AP)
Israel has shown interest in the vaccine since Russia announced it was ready for human trials, and in early November the director of Jerusalem’s Hadassah Medical Center said the hospital had ordered 1 , 5 million units of vaccine and would seek approval from the Ministry of Health for their use, pending phase 3 trials.

A Russian medical worker administers a photo of the Russian experimental vaccine against the Sputnik V coronavirus in Moscow, Russia, September 15, 2020 (Alexander Zemlianichenko Jr / AP)
The Hadassah Medical Center in Moscow, a branch of the Israeli Hospital, is involved in the administration and monitoring of the Phase 3 vaccine trial. Jerusalem Hadassah Medical Center CEO Zeev Rotstein said Russian authorities asked Hadassah to file documents for vaccine approval with Israel’s health ministry.
Russia registered the vaccine for public use in August, in an unusual move, ahead of the start of phase 3 trials in September. The phase 3 trial involved 40,000 volunteers at 29 clinics in Moscow. A quarter received a placebo. In addition, 10,000 people considered to be at high risk of the virus were also inoculated.
Requests for more than 1.2 billion doses of the Sputnik V vaccine have arrived so far from more than 50 countries, RDIF said.
A vaccine is seen as the best hope to break the cycle of deadly virus outbreaks and severe restrictions across much of the world since COVID-19 first emerged in China late last year and has devastated the world economy.
So far, 1.2 million people have died from COVID-19, the disease caused by the virus.
Israel is also working on a local vaccine, although it is currently only in Phase 1 trials and its development is expected to take months longer than foreign applicants.
[ad_2]
Source link