
[ad_1]
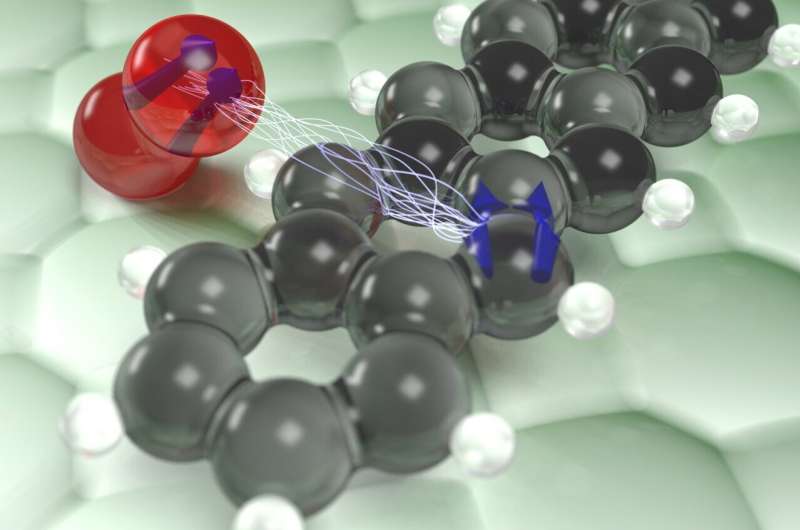
Artist’s impression of the interaction of the triplet state (blue arrows) of an individual pentacene molecule (black and white) with an oxygen molecule (red). Credit: Jascha Repp
Researchers at the University of Regensburg are following the first step in the reaction of a single coloring pigment with oxygen at unprecedented resolution.
Why do the colors of a t-shirt fade over time in the sun? Why do you get sunburned and why do the leaves on a tree turn brown in the fall? These questions all have one theme in common: the interaction between coloring pigments and ambient oxygen. Every child learns about this chemical reaction in school, which is the process of oxidizing the air we breathe. So what could be left to research?
Oxygen is an amazing molecule in that it is magnetic. In liquid form, at very low temperature, it can be picked up by a magnet just like iron filings. This property is related to the electrons in oxygen. All molecules are made up of atomic nuclei and electrons, which behave like tiny needles on a compass. Usually, these needles are arranged in pairs pointing in opposite directions so that their magnetic forces cancel each other out. In an oxygen molecule made up of two oxygen atoms, however, the two compass needles point in the same direction, making the oxygen magnetic.
Dye molecules, such as those used to color a t-shirt, are not magnetic because the needles of the electron compass point in opposite directions. When light shines on such a molecule, a certain color of the light will be absorbed, giving the dye its characteristic appearance. In this process of absorbing light, the energy of light is transferred to an electron in the dye molecule, breaking the original pairing of two electrons and allowing the needle of the excited electron’s compass to spontaneously change its alignment. When this process occurs, the electron can no longer return to its original state. The dye molecule becomes magnetic, entering what is called a “triplet state”.
An international research team led by Professor Jascha Repp has now succeeded in revealing how this triplet energy is transferred from a single dye molecule to a single oxygen molecule. This process is an integral part of everyday life, where many oxidation reactions take place via the excited triplet state. As long as the molecule resides in this state, it retains the energy transmitted to it by light, thus facilitating chemical reactions. Most chemical reactions, such as combustion, require some initial energy such as a spark to start.
Complete dissipation of energy within the dye molecule requires another reversal of the alignment of the electronic compass needle, which is a slow and unlikely process. Alternatively, the light energy inside the dye molecule, which is magnetic energy, can simply be transferred to another magnetic molecule, such as oxygen, a process similar to flipping a bar magnet into spinning another nearby. This energy transfer de-energizes the dye molecule, but it tends to make the oxygen molecule itself very reactive, ultimately destroying the dye molecule. This effect can be seen on bleached t-shirts or sunburns, where the dye molecules are the pigments in the skin.
The team was able to track this energy transfer between the dye and the oxygen molecule directly in space, without destroying the dye molecule. To do this, isolated molecules were placed on a surface and cooled to very low temperatures close to that of the universe. Using an “atomic force microscope” made up of a very fine needle with a single atom at its tip, the researchers were able to image the individual atoms of the dye molecule by scanning the tip through it. By applying a sequence of electrical pulses to the dye molecule, they could drive it to the magnetic triplet state in a controlled manner. The transfer of energy from this triplet excited state to nearby oxygen molecules was then tracked over time by measuring tiny changes in the force acting on the tip.
This new approach, reported in Science, allowed researchers to probe many different geometries of the arrangement of the dye molecule and oxygen. In this way, the interplay between molecular arrangements at the atomic level and the rate at which such energy transfer occurs could be resolved for the first time. Scientists now aim to be able to finally formulate an underlying microscopic framework of fundamental oxidation reactions. In addition to the annoying fading of t-shirts, such an interaction between molecular triplet excitations is of utmost importance for a series of technological developments, such as in organic light-emitting diodes (OLEDs) and organic solar cells, in the photocatalytic energy conversion and photosynthesis and in the photodynamic therapy of cancer.
Chemists discover new fluorescence in carbon nanotubes
Atomically resolved monomolecular triplet quenching, Science (2021). science.sciencemag.org/cgi/doi… 1126 / science.abh1155
Provided by the University of Regensburg
Quote: New microscope reveals molecular oxygen miracle (2021, July 22) retrieved July 23, 2021 from https://phys.org/news/2021-07-microscope-reveals-miracle-molecular-oxygen.html
This document is subject to copyright. Other than fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.
[ad_2]
Source link