
[ad_1]
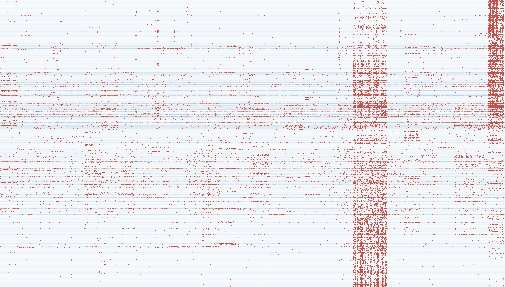
Thermal map illustrating the expression of transcripts specific to melanoma in various types of cancer. The three high-expression columns come from melanoma samples. Credit: Francis Crick Institute
According to new research from Crick's scientists, the "echoes" of DNA from viruses that infected our ancestors millions of years ago could help the immune system identify and kill cancer cells.
The new study, published in Genome research, examined "endogenous retroviruses", fragments of DNA from the human genome left behind by viruses that infected our ancestors.
For millions of years, our ancestors have been infected with innumerable viruses and their DNA is now more our genome than human genes. About 8% of the human genome is made up of retroviral DNA, whereas known genes only represent 1 to 2%.
"This viral DNA is usually dormant because it is non-functional or our body has evolved to remove it," says Dr. George Kassiotis, head of the Crick group, who led the study. "However, when a cell becomes cancerous, some of these deletion mechanisms may fail and this old viral DNA may be reactivated." In this study, we looked for cancer-reactivated viral DNA and producing products. that the immune system can see, the hope is that if we can train the immune system to detect them, we can selectively target the cancer cells. "
Revival of ancient DNA
Genes are DNA fragments containing instructions for the production of proteins, which perform important functions in the cell or body. These instructions are transcribed into "messenger" molecules of RNA before the production of proteins. However, this transcription process may be influenced by DNA located outside the gene, including endogenous retroviruses.
To study the effects of endogenous retroviruses on transcription, the team examined patient samples from 31 different cancer types using a technology called "RNASeq" that can read short, random fragments of RNA. However, each "read" only provides a small portion of the sequence in an unknown order, it takes up to 50 million "reads" per sample to get a complete picture of the transcription activity.
"Assembling together a complete transcriptional profile is a monumental task," George said. "It's been compared to trying to read a magazine that has been shredded into millions of pieces, when you do not even know what it's supposed to be about or what language it's in. All you have are fragments random, so to cut them together, you have to see where they overlap. "
The team used the RNA sequencing data from 768 patient samples, with nearly 40 billion reads to be reconstructed. Even using sophisticated algorithms, a desktop computer should run continuously for 24 years to assemble this data. To speed things up significantly, the researchers called upon Crick's specialized computer science team. By performing the analysis on the High Performance Computing internal cluster, they achieved results much more quickly.
Close on cancer
From the complete transcription data, the team developed a catalog of over 130,000 different RNA transcripts produced by endogenous retroviruses, more than half of which had not been discovered before. Of these, approximately 6,000 transcripts were specifically found in cancer samples and not in healthy tissue. Many of them were specific to the type of cancer, with most cancers expressing high levels of a few hundred transcripts.
"We focused on melanoma-specific transcripts and applied an algorithm to predict which one could code for visible material for the immune system," says George. "We found 14 candidate transcripts from 8 different regions of the genome that could produce unique cancer antigens." Together with the Proteomics team at Crick Lab and Nicola Ternette in Oxford, we looked at spectrometry data from mass to determine which of these antigens were actually present. " We hoped that this approach could form the basis of future cancer treatments, if we can vaccinate the immune system to recognize and attack the cancer cells with these peptides. "
George is one of the scientific co-founders of Ervaxx, a spin-off company of the Crick Group that aims to introduce this science into a clinic to help patients. Ervaxx builds on this fundamental information to create a portfolio of standard, cancer-specific vaccines and other immunotherapies, including a flagship product candidate for the treatment of patients with melanoma.
A carcinogenic mutation suppresses the immune system around tumors
Jan Attig et al. Retro-active LTR expansion of human cancer transcriptome and immunopeptidome revealed by de novo transcript assembly, Genome research (2019). DOI: 10.1101 / gr.248922.119
Quote:
Old viruses could help kill cancer (September 20, 2019)
recovered on September 21, 2019
from https://medicalxpress.com/news/2019-09-ancient-viruses-cancers.html
This document is subject to copyright. Apart from any fair use for study or private research purposes, no
part may be reproduced without written permission. Content is provided for information only.
[ad_2]
Source link