[ad_1]
After a review surfaced on the data behind Moderna’s COVID-19 coup on Tuesday, many have since caught several reported incidents of Bell’s palsy, or facial palsy, among those vaccinated in the trial.
Documents released by a U.S. Food and Drug Administration committee cited three incidents of facial paralysis among Moderna vaccinees and one in the placebo group. However, due to limited data, “a causal relationship with vaccination cannot be concluded at this time,” the FDA said.
A senior expert on Bell’s palsy at Johns Hopkins Medicine recently told Fox News that the incidence of facial palsy in trial participants is actually lower than what happens naturally, and said that Bell’s palsy could not be directly attributed to COVID-19 vaccines.
FDA ‘WORKING QUICKLY’ TO FINALIZE MODERNA COVID-19 VACCINE APPROVAL
Additionally, FDA documents posted before the now-licensed Pfizer vaccine reported multiple cases of Bell’s palsy in the vaccine group, although none in the placebo group and the FDA wrote: “All four cases in the vaccine group do not represent a higher frequency than expected in the general population. “These cases occurred 3, 9, 37 and 48 days after vaccination.
Nonetheless, the FDA has advised monitoring Bell’s palsy among recipients of the Pfizer and Moderna vaccines, the latter awaiting formal approval from the federal agency, during administration to the masses.
Usually, the exact cause of Bell’s palsy is unknown and it usually occurs on one side of the face, although it can occur on both sides on rare occasions, according to the National Institutes of Health. Although symptoms vary in severity, they usually improve within a few weeks, and approximately 40,000 people are affected by this type of paralysis in the United States each year.
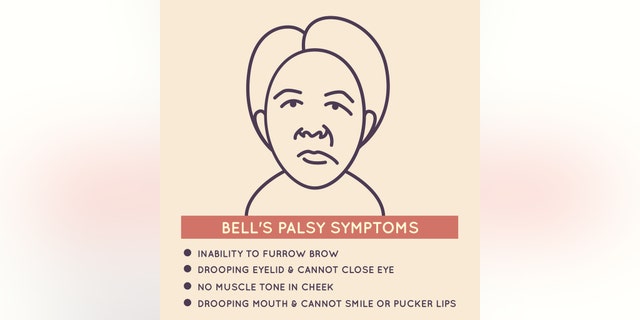
Dr Kofi Boahene, head and neck surgery expert at Johns Hopkins Medicine, told Fox News that the incidence of Bell’s palsy is between 15 and 30 cases per 100,000 people. He said facial paralysis in trial participants could not be directly related to the vaccine.
“By extrapolation, the calculated incidence of Bell’s palsy among the two vaccine studies is lower than what occurs naturally,” Boahene wrote to Fox News. “I would agree that the occurrence of Bell’s palsy among vaccinated volunteers cannot be directly attributed to the vaccine.”
It should be noted, as previously reported, that the independent FDA committee did not find any specific safety issues associated with Moderna’s vaccine, and that Pfizer’s product has already passed the approval process required for authorization. emergency. Healthcare workers who have occupied the coronavirus frontlines for 10 months continue to queue for their vaccinations, the first of which was rolled out on Monday. Residents of nursing homes have also started to get vaccinated, as have some government officials.
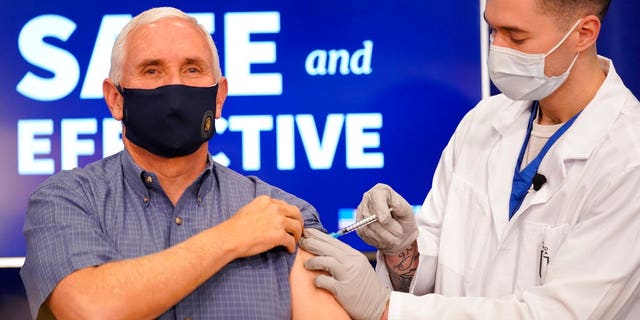
Vice President Mike Pence receives a Pfizer-BioNTech COVID-19 vaccine injected into the Eisenhower Executive Office building on the White House complex on Friday, December 18, 2020 in Washington. Karen Pence and US Surgeon General Jerome Adams also participated. (AP Photo / Andrew Harnik)
PENCE GETS COVID-19 VACCINE TO SHOW ‘SAFE AND EFFECTIVE’
The cases of Bell’s palsy reported among participants in the Moderna trial occurred 22, 28, and 32 days after vaccination, with the patient who received a placebo exhibiting facial palsy 17 days after injection. At least three-quarters of these cases were reported as “resolvable” in FDA documents.
One of the cases involved a 67-year-old diabetic patient hospitalized for a stroke. About a month after being vaccinated, she experienced ‘new facial paralysis’, although she was reported to be ‘in the process of resolution’.
Another patient who received Moderna’s vaccine was a 30-year-old man who developed facial paralysis 28 days after vaccination, with a coincident upper respiratory infection, although this was reported to be resolved.
Finally, among the vaccinated group, an elderly patient also developed facial paralysis 22 days after vaccination, reported as ongoing at the time of a safety report submitted at the end of November.
GET THE FOX NEWS APP
The fourth case of “resolution” occurred in a 52-year-old male patient who received not the vaccine, but the placebo, 17 days after infection.
Boahene, the Johns Hopkins expert, said Bell’s palsy occurs at all ages and while some risk factors may be predisposing, “none are apparent among the reported cases.”
“The history, age and timing between injection and onset of Bell’s palsy [does not] lend to specific conclusions about cause and effect, ”he said.
[ad_2]
Source link