[ad_1]
Latest medical innovation proven to give better treatment during the onset of breast cancer
(philstar.com) – November 29, 2018 at 10:18 am
MANILA, Philippines – Breast cancer is the most common cancer in women and its incidence is the highest in the world. Every year, more than 1.4 million new cases are diagnosed worldwide and more than 450,000 women will die from the disease each year. In the Philippines, in 2018, breast cancer was the most prevalent cancer in the country, accounting for more than 19% of newly reported cases.
As part of the recently concluded agreement with the Philippine Society of Medical Oncology (PSMO), organized in partnership with the American Society of Clinical Oncology (ASCO), the Roche Pharmaceutical Company (Philippines) Inc. shared the latest innovations in breast cancer treatment HER2-positive blocking treatments.
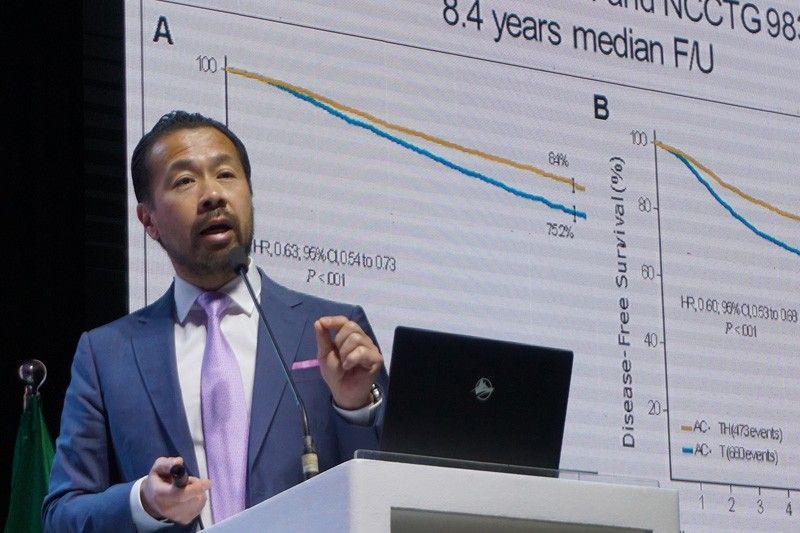
Dr. Stephen Chia, a professor in the Department of Medicine at the University of British Columbia in Vancouver, Canada, and chair of the Breast Tumor Group of British Columbia, presented the latest research on unmet need of patients with HER2-positive breast cancer in adjuvant setting. . He showed the results of clinical trials using pertuzumab in adjuvant combination, allowing for a shorter recurrence of the disease in patients with early-stage HER2-positive breast cancer.
HER2-positive breast cancer
Human breast cancer with human epidermal growth receptor (HER2) is a type of breast cancer overexpressing a protein called HER2.
Of women with breast cancer, 15 to 20% are HER2-positive. This type of breast cancer tends to be more aggressive than other types. HER2-positive patients are also less sensitive to hormones or other forms of initial treatment. However, treatments specifically targeted for HER2 can be very effective.
Many patients with HER2-positive breast cancer can be treated with surgery, chemotherapy or targeted therapies to achieve positive results.
Adjuvant treatment of pertuzumab with trastuzumab
"In the time before trastuzumab, HER2-positive breast cancer was a subgroup that had the worst prognosis," said Dr. Chia.
"With chemotherapy and adjuvant treatment with trastuzumab, the results have been significantly improved. However, he still has not healed all women. We still see about 25% of breast cancer patients with a (+) HER2-positive node who relapsed 10 years after the standard care of adjuvanted trastuzumab for one year. So there is always an unmet need, "he said.
Dr. Chia shared the results of the latest clinical trial – APHINITY. In the study, patients received pertuzumab in addition to the standard care of adjunctive treatment with trastuzumab for one year. The results showed a significant improvement in reducing the risk of disease recurrence or death. In addition, no new security signal was issued following the treatment.
"This study resulted in a statistically significant improvement in survival without invasive disease in patients," said Dr. Chia. "The control groups, which received standard chemotherapy and adjuvant therapy with Trastuzumab alone, again performed well and had a disease-free survival rate of nearly 91%. This has been improved by about 20% with the addition of pertuzumab. "
In the Philippines, pertuzumab is approved for the treatment of early breast cancer and HER2-positive metastatic breast cancer in patients eligible for treatment through diagnostic tests (ie HER2 test). Pertuzumab is a prescription medication that works synergistically with trastuzumab to block the survival and growth signals of cancer cells, specifically targeting the HER2 receptor.
The combination of treatments with pertuzumab and trastuzumab provides longer overall survival in patients with HER2-positive breast cancer.
To help make pertuzumab treatment more accessible to Filipino patients, Roche has created an innovative program to assist patients.
For more information and to register, doctors and patients can contact (02) 395-3558.
[ad_2]
Source link