
[ad_1]
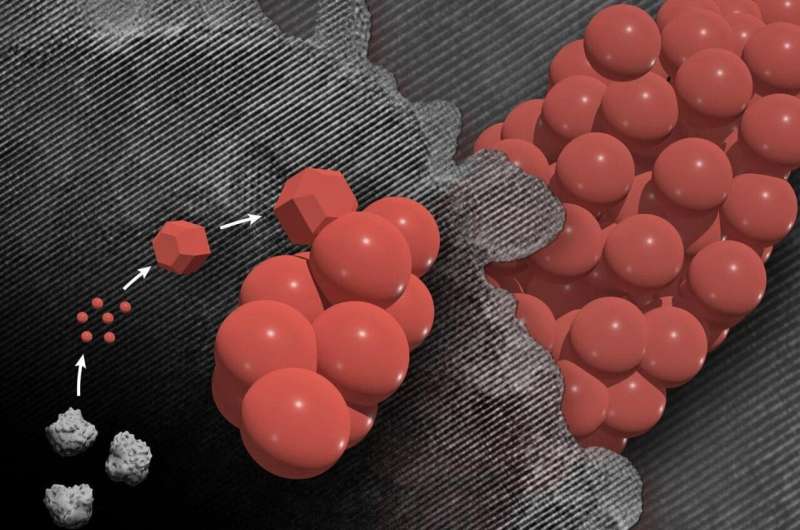
The researchers used advanced microscopy techniques to observe the formation of mesocrysts in real time. Credit: Composite image by Mike Perkins | Pacific Northwest National Laboratory
When materials reach extremely small size scales, strange things start to happen. One of these phenomena is the formation of mesocrystals.
Although they are made up of separate individual crystals, the mesocrystals come together to form a larger fused structure that behaves like a pure single crystal. However, these processes occur at scales far too small for the human eye to see and their creation is extremely difficult to observe.
Because of these challenges, scientists had not been able to confirm exactly how mesocrysts are formed.
New research by a team led by the Pacific Northwest National Laboratory (PNNL) used advanced transmission electron microscopy (TEM) techniques to watch mesocrysts form in solution in real time. What they saw goes against conventional wisdom, and their ideas could one day help scientists design materials for energy storage and understand how minerals form in soil.
Rather than the nucleation of individual crystals, the step that begins crystal formation and then random aggregation into unrelated two-step mesocrystals, the researchers observed that nucleation and attachment were tightly coupled to form these highly uniforms. The researchers reported on their work in the February 18, 2021 issue of Nature.
“Our results identify an important new pathway for crystallization through particle attachment and resolve key questions about mesocrystals formation,” said Guomin Zhu, materials scientist at PNNL and the University of Washington. He was part of the research team led by Jim De Yoreo, materials scientist at PNNL and co-director of the Northwest Institute for Materials Physics, Chemistry, and Technology. “We suspect this is a widespread phenomenon with significant implications both for the synthesis of engineered nanomaterials and for understanding natural mineralization,” Zhu added.
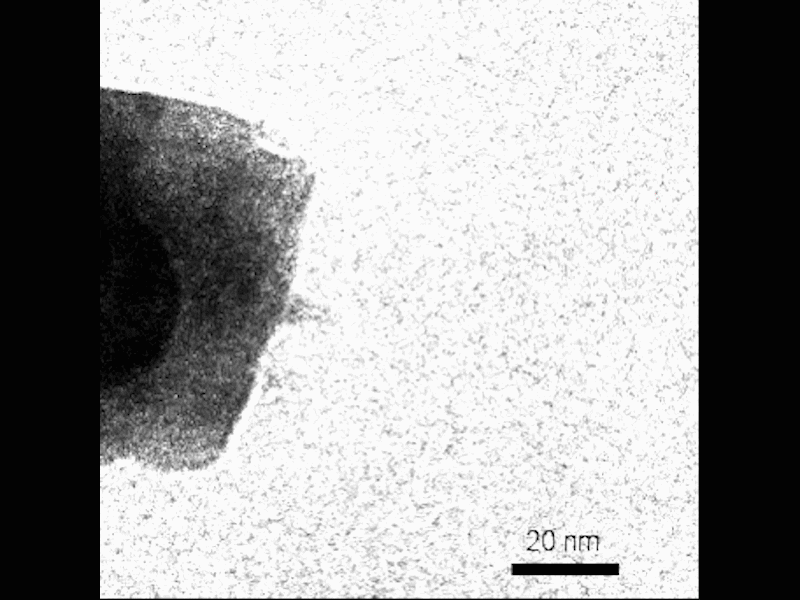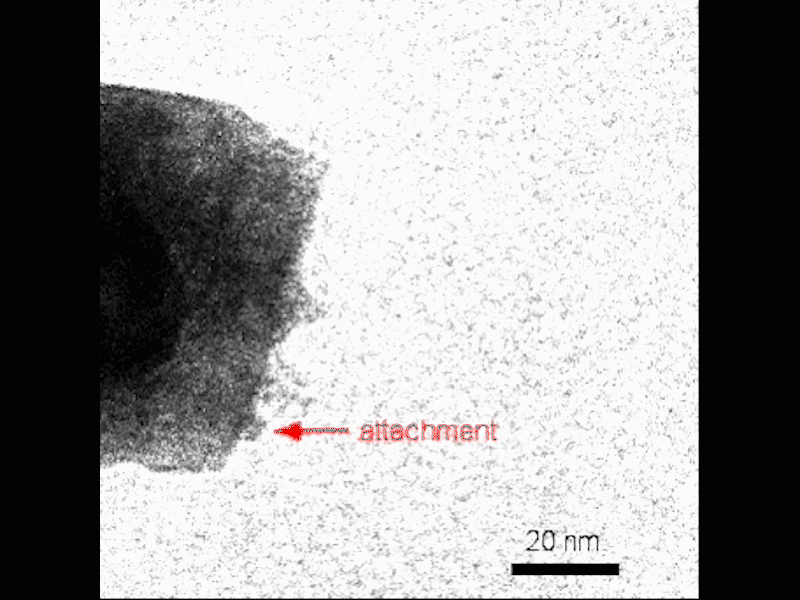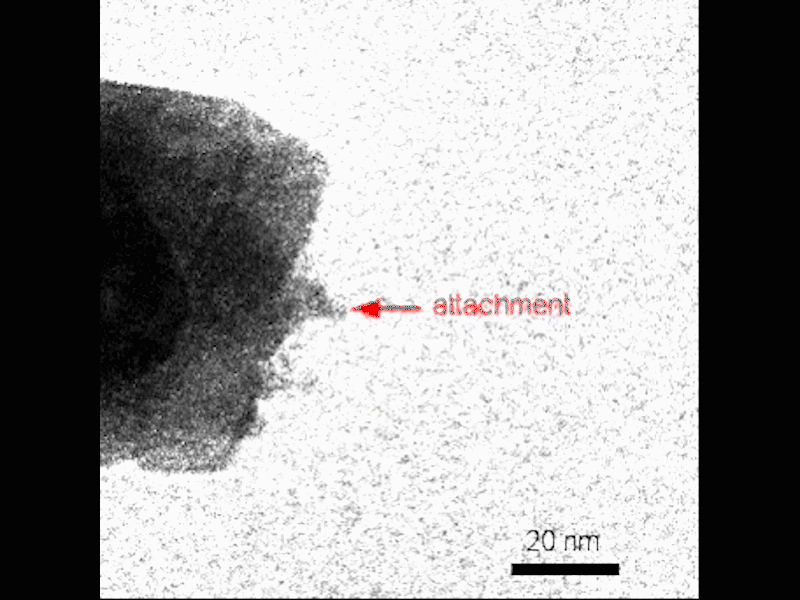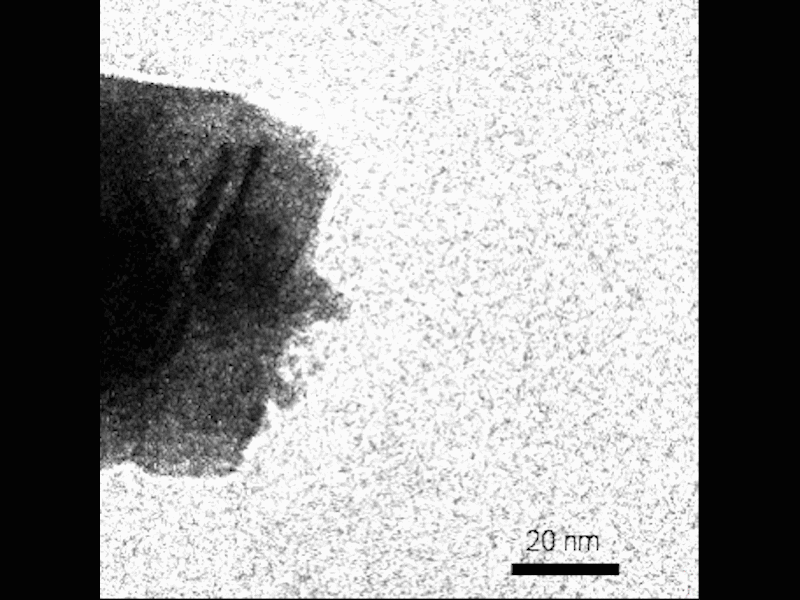
Observe the nucleation of small crystals near the surface of the growing mesocryst before attachment. Credit: Video by Guomin Zhu | Pacific Northwest National Laboratory
See crystallization in real time
The project took years to complete and required significant problem solving. For the microscopy experiments, the science team chose a model system that included hematite, an iron compound commonly found in the earth’s crust, and oxalate, a compound naturally abundant in soil.
They visualized the process using TEM in situ, which gives researchers the ability to see nanoscale crystallization as it goes. They combined this real-time method with a “gel-and-look” TEM which allowed them to follow an individual crystal at different points during growth. Theoretical calculations helped complete the picture, allowing the PNNL team to reconstruct the growth of the mesocrysts.
Researchers typically run most TEM experiments in situ at room temperature to simplify the experimental setup and minimize the potential for damaging the sensitive instrument, but the formation of mesocrysts fast enough to be observed occurs at around 80 ° C.
“The additional equipment used to heat the samples made the experiments extremely difficult, but we knew the data would be critical in understanding how mesocrysts formed,” Zhu said.
When heated, the new hematite nanocrystals facilitate their rapid attachment, which leads, on average, to final mesocrysts of approximately the same size and shape.

A transmission electron microscope allows researchers to observe the fundamental processes of crystal formation. Credit: Photo by Andrea Starr | Pacific Northwest National Laboratory
Mesocrysts in nature
The chemical key to this fast and reliable fixation lies in the oxalate molecules present in the solution. After the first small crystals form, the oxalate additives help create a chemical gradient at the interface of the liquid and the growing crystal. More of the chemicals needed for particle nucleation persist near the crystals, which greatly increases the likelihood of new particles forming near existing ones.
While this pathway for crystal growth has been observed under controlled conditions at very small scales, it likely occurs in natural systems as well, the researchers said. Some mineral deposits, including an Australian hematite deposit, contain mesocrystals. Given the natural abundance of oxalate and the PNNL team’s observation that hematite can become mesocrystals at temperatures as low as 40 ° C, it seems plausible that this formation pathway occurs. occur in nature.
Since mesocrystals are found in nature, the results can be applied to understanding the cycling of nutrients in the environment, among other applications. In addition, to create complex structures that are almost uniform, one must understand how the methods of forming these materials work and how to control them. So, this work, supported by the US Department of Energy, Office of Science, Office of Basic Energy Sciences, Division of Chemical Sciences, Geosciences, and Biosciences, opens up new possibilities for intentionally creating mesocrysts or materials from mesocrystal type.
Highly efficient hydrogen gas production using sunlight, water and hematite
Self-similar mesocrystals are formed by nucleation and assembly driven by the interface, Nature (2021). DOI: 10.1038 / s41586-021-03300-0, dx.doi.org/10.1038/s41586-021-03300-0
Provided by Pacific Northwest National Laboratory
Quote: Researchers discover new way to form complex crystals (2021, February 17) retrieved February 19, 2021 from https://phys.org/news/2021-02-route-complex-crystals.html
This document is subject to copyright. Other than fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.
[ad_2]
Source link