
[ad_1]
Researchers at the Hebrew University of Jerusalem said they have found a way to turn skin cells into three main types of stem cells that include early-stage embryos.
The discovery could pave the way for the creation of whole human embryos from human skin cells, without requiring sperm or eggs, the researchers said. And this could also have "broad implications" for modeling embryonic defects and highlighting placental dysfunctions, as well as for solving some infertility problems by creating human embryos in a petri dish, said a statement from the Hebrew University.
"You could say we're about to generate a synthetic embryo, which is really crazy," said Dr. Yossi Buganim of the Department of Developmental Biology and Cancer Research at the university, which has led the study published in Cell Stem Cell.
Receive by email the Start-Up Daily Israel Start-Up and never miss our best stories
Free registration
This discovery could in the future allow researchers "to generate embryos of men and sterile women, using only their skin cells, and to generate a real embryo in a box and implant it. the embryo in the mother, "said Buganim in a telephone interview.
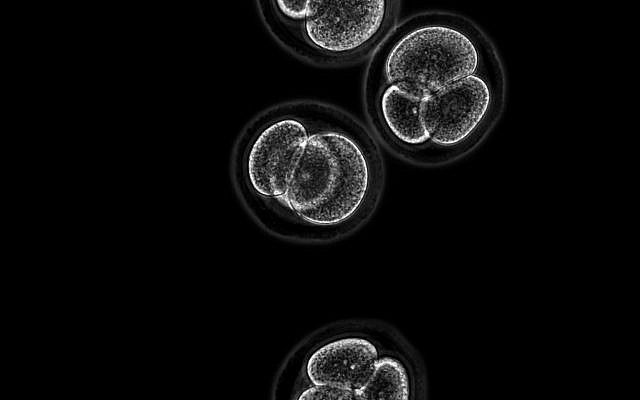
Researchers at the Hebrew Hebrew University of Jerusalem have found a way to turn skin cells into three main types of stem cells including early-stage embryos; Image shows 4-cell stage mouse embryos (Kirill Makedonski)
Buganim and his team have discovered a set of five genes capable of transforming murine skin cells into three types of cells constituting the early embryo: the fetus itself, the placenta and the extra-embryonic tissues, such as the cord umbilical.
In 2006, Japanese researchers Kazutoshi Takahashi and Shinya Yamanaka discovered the ability to "reprogram" skin cells into early embryonic cells that can generate an entire fetus through the use of four central embryonic genes. These genes reprogrammed skin cells into "induced pluripotent stem cells" (iPSCs), which resemble cells that develop shortly after fertilization and are essentially identical to their natural counterparts. These cells can grow in all types of fetal cells, but not in extraembryonic tissues, such as the placenta.
Japanese researchers have discovered that the four central embryonic genes can be used to "rejuvenate" skin cells so that they function as embryonic stem cells, Buganim explained.
After fertilization of the egg, the cell divides into 64, creating a bowl of cells that constitute the three crucial parts of an embryo – the epiblast, the inner cell mass that gives birth to the fetus ; the primitive endoderm responsible for the umbilical cord; and a third part, the trophectoderm, responsible for the creation of the placenta.
What the Japanese managed to do, Buganim said, was to turn skin cells into fetal stem cells. But this is not enough to create an entire embryo, he said, because the other parts are also needed – those that develop the umbilical cord and the placenta.

Dr. Yossi Buganim, Department of Developmental Biology and Cancer Research, Hebrew University (Shai Herman)
The breakthrough of the Hebrew University team, said Buganim, was to create with five genes the three essential compartments that make up the embryonic and extra-embryonic features needed to create an embryo in vitro. The work was done with mice and the team is now starting to apply the same research to human embryos, he added.
The researchers used five genes completely different from those used by Japanese researchers, noted Buganim. The genes used by Israeli researchers are those that play a role in the early development of the embryo. They specify and direct the development of each cell, be it the umbilical cord, the placenta or the fetus itself.
The team used a new technology to study the molecular forces that dictate the development of each cell. For example, the researchers found that the "Eomes" gene pushed the cell towards the identity and placental development of stem cells, while "Esrrb" orchestrated the development of fetal stem cells, by obtaining the same effect. first, but only temporarily, extra-embryonic stem cell identity. .
"We had the idea of using these genes," Buganim said.
The researchers then combined these five genes so that, once inserted into skin cells, they successfully reprogrammed the cells into each of three early embryonic cell types from the same petri dish.
This discovery will enable researchers to better understand and treat embryonic dysfunctions and diseases such as placental insufficiency or miscarriage, he said. This could allow researchers to use a dish to model embryonic cells and identify early risk markers.
Still a long way to go
The challenges ahead are still huge, said Buganim. "An embryo is a three-dimensional structure. We must learn to put all of this together to generate a real embryo. We need to identify the ratios "of placental stem cells, umbilical cord cells and iPS cells, which create the fetuses, and in what" scaffolding "to place them, he said.
"These cells know how to stay together," Buganim said. "I have to give them the right environment and the right ratio to organize them into a real embryo."
The study was conducted by Buganim in collaboration with Dr. Oren Ram of the Institute of Life Sciences of the Hebrew University and Professor Tommy Kaplan of the Faculty of Informatics and Engineering of the University, as well as as doctoral students Hani Benchetrit and Mohammad Jaber.
[ad_2]
Source link