[ad_1]
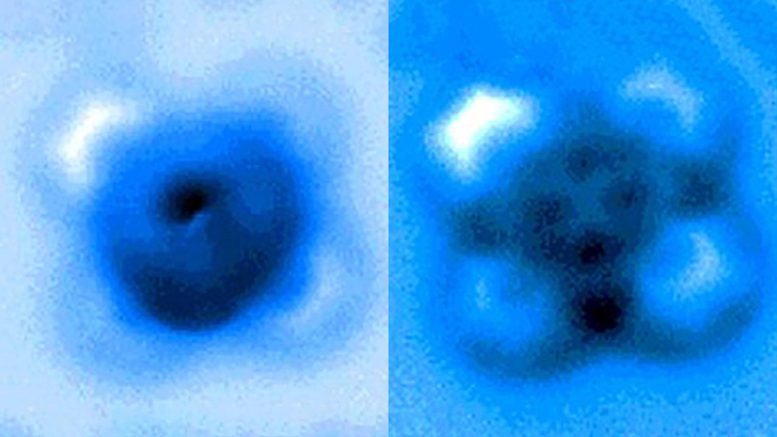
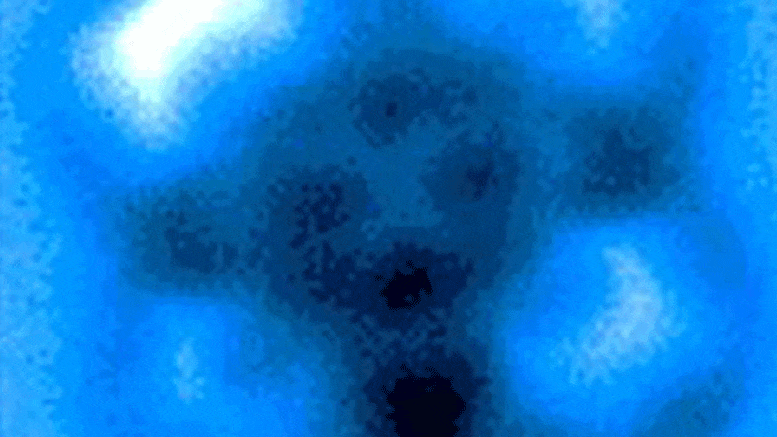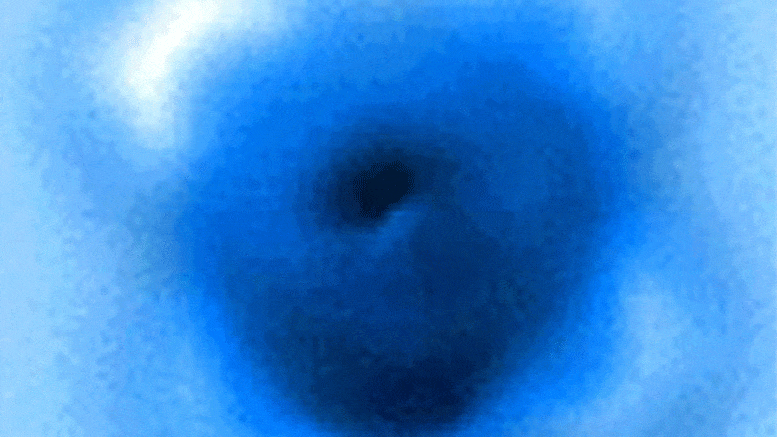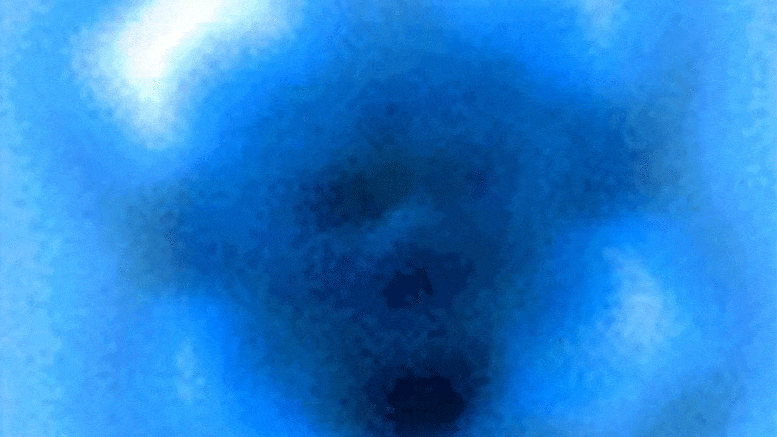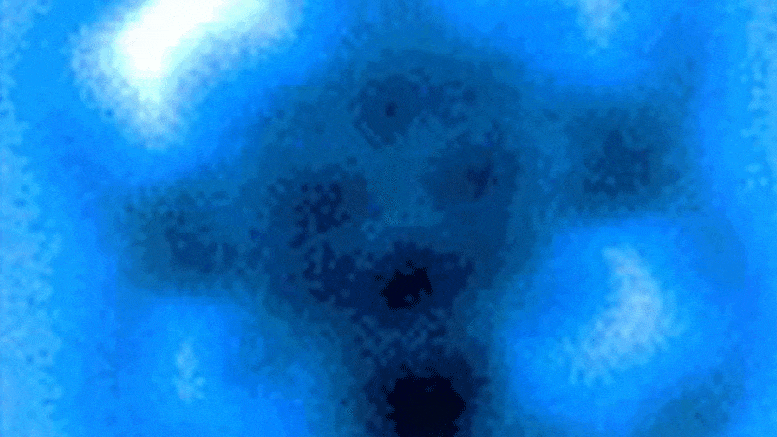
The researchers measured the mechanical forces applied to break a bond between carbon monoxide and iron phthalocyanine, which appears as a symmetrical cross in scanning probe microscope images taken before and after the rupture of the connection. Credit: Pengcheng Chen et al.
Using advanced microscopy techniques to Princeton University, researchers have recorded the breaking of a single chemical bond between a carbon atom and an iron atom on different molecules.
The team used a high-resolution atomic force microscope (AFM) operating in a controlled environment at the Princeton Imaging and Analysis Center. The AFM probe, whose tip ends with a single copper atom, was gradually brought closer to the iron-carbon bond until it broke. The researchers measured the mechanical forces applied at the time of failure, which was visible in an image captured under a microscope. A team from Princeton University, the University of Texas-Austin and ExxonMobil reported the results in an article published on September 24, 2021 in Nature Communication.
“It’s an incredible picture – to be able to see a single small molecule on a surface with another linked to it is incredible,” said co-author Craig Arnold, Susan Dod Brown professor of mechanical and aerospace engineering and director from the Princeton Institute for Materials Science and Technology (PRISM).
“The fact that we can characterize this particular link, both by pulling on it and pushing it, allows us to better understand the nature of these types of links – their strength, their interaction – and that has all kinds of implications, in especially for catalysis, where you have a molecule on a surface and then something interacts with it and causes it to break down, ”Arnold said.
Nan Yao, principal investigator of the study and director of the Princeton Imaging and Analysis Center, noted that the experiments also revealed how bond breaking affects a catalyst’s interactions with the surface on which it is. absorbed. The improved design of chemical catalysts is relevant to biochemistry, materials science and energy technologies, added Yao, who is also a practicing professor and principal investigator at PRISM.
In the experiments, the carbon atom was part of a carbon monoxide molecule, and the iron atom came from iron phthalocyanine, a common pigment and chemical catalyst. Iron phthalocyanine is structured like a symmetrical cross, with a single iron atom at the center of a complex of connected rings based on nitrogen and carbon. The iron atom interacts with the carbon of carbon monoxide, and iron and carbon share a pair of electrons in a type of covalent bond known as a dative bond.
Yao and his colleagues used the AFM instrument’s atomic-scale probe tip to break the iron-carbon bond by precisely controlling the distance between the tip and bound molecules, down to 5 picometer increments. (5 billionths of a millimeter). The break occurred when the tip was 30 picometers above the molecules – a distance that is about one-sixth the width of a carbon atom. At this height, half of the iron phthalocyanine molecule became more blurry in the AFM image, indicating the point at which the chemical bond was broken.
The researchers used a type of AFM known as non-contact, in which the tip of the microscope does not directly contact the molecules being studied, but instead uses changes in the frequency of small-scale vibrations to construct an image of the surface of the molecules.
By measuring these frequency shifts, the researchers were also able to calculate the force needed to break the bond. A standard copper probe tip broke the iron-carbon bond with an attractive force of 150 piconewtons. With another carbon monoxide molecule attached to the tip, the bond was severed by a repulsive force of 220 piconewtons. To deepen the basis for these differences, the team used quantum simulation methods to model changes in electron densities during chemical reactions.
The work takes advantage of advanced AFM technology for the first time in 2009 to visualize single chemical bonds. Controlled breaking of a chemical bond using an AFM system was more difficult than similar studies of bond formation.
“It is a great challenge to improve our understanding of how chemical reactions can be carried out by manipulating atoms, i.e. with the tip of a scanning probe microscope,” said Leo Gross, who heads the Atom and Molecule Manipulation research group at IBM. Research in Zurich, and was the lead author of the 2009 study that first solved the chemical structure of a molecule by AFM.
By breaking a particular bond with different tips that use two different mechanisms, the new study helps “improve our understanding and control of bond cleavage by manipulation of atoms.” It adds to our toolkit for atom-manipulated chemistry and represents a step forward in manufacturing engineered molecules of increasing complexity, ”added Gross, who was not involved in the study.
Experiences are extremely sensitive to external vibrations and other confounding factors. The specialized AFM instrument at the Imaging and Analysis Center is housed in a high vacuum environment, and materials are cooled to a temperature of 4 Kelvin, a few degrees above absolute zero, using liquid helium. These controlled conditions give precise measurements by ensuring that the energy states and interactions of molecules are only affected by experimental manipulations.
“You need a really good, clean system because this reaction can be very complicated – with so many atoms involved, you might not know which bond you are breaking on such a small scale,” Yao said. “The design of this system simplified the whole process and clarified the unknown” by breaking a chemical bond, he said.
Reference: “Breaking a Dative Bond with Mechanical Forces” by Pengcheng Chen, Dingxin Fan, Yunlong Zhang, Annabella Selloni, Emily A. Carter, Craig B. Arnold, David C. Dankworth, Steven P. Rucker, James R. Chelikowsky and Nan Yao, September 24, 2021, Nature Communication.
DOI: 10.1038 / s41467-021-25932-6
The lead authors of the study were Pengcheng Chen, associate researcher at PRISM, and Dingxin Fan, a Ph.D. student at the University of Texas-Austin. In addition to Yao, other corresponding authors were Yunlong Zhang of ExxonMobil Research and Engineering Company in Annandale, New Jersey, and James R. Chelikowsky, professor at UT Austin. Besides Arnold, the other Princeton co-authors were Annabella Selloni, professor of chemistry David B. Jones, and Emily Carter, professor Gerhard R. Andlinger ’52 in energy and environment. The other ExxonMobil co-authors were David Dankworth and Steven Rucker.
This work was supported in part by ExxonMobil through its membership in the Princeton E-ffiliates Partnership of the Andlinger Center for Energy and the Environment. Princeton University’s Center for Imaging and Analysis is supported in part by the Princeton Center for Complex Materials, a materials science and engineering research center of the National Science Foundation. Additional support was provided by the Welch Foundation and the US Department of Energy.
[ad_2]
Source link