
[ad_1]
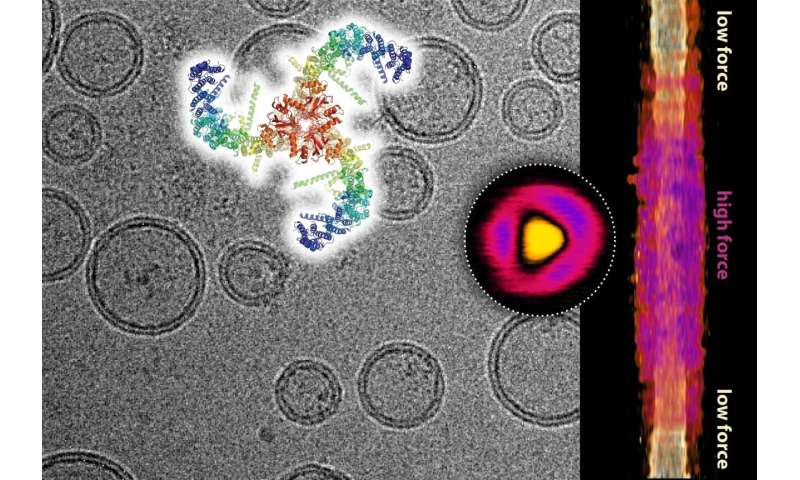
Composite image in cryogenic microscopy showing lipid vesicles with incorporated piezo channels and the structure of the piezo channel (top). Topographic image of a single piezo channel under force recorded by high-speed atomic force microscopy (circle) and its lateral expansion in the membrane as a function of the force applied (right). Credit: Dr. Simon Scheuring
A team of scientists from Weill Cornell Medicine and Rockefeller University has shed light on the basic mechanism of piezoelectric proteins, which serve as sensors to the body for mechanical stimuli such as touch, bladder and pressure blood. This discovery is a feat of basic science that also opens up many new avenues for investigation of the role of piezo proteins in human diseases and potential new therapeutic strategies.
In the study, published August 21 in Nature, scientists have used advanced microscopy techniques to image Piezo1 protein at rest and during the application of mechanical forces. They confirmed the structure of this complex protein and essentially showed how it can convert mechanical stimuli into an electrical signal.
"Our analysis shows that the tension on the cell membrane in which Piezo1 is integrated can flatten and expand the structure of the protein," said senior co-author Dr. Simon Scheuring, professor of physiology and biophysics in Weill anesthesiology. Cornell Medicine. Dr. Scheuring and his lab collaborated in the study with the laboratory of Dr. Roderick MacKinnon, Professor of Molecular Neurobiology and Biophysics at Rockefeller University. Dr. MacKinnon was co-recipient of the 2003 Nobel Prize in Chemistry for his work on the determination of ion channel protein structures and mechanisms.
Piezo1 and Piezo2 are very large and complex proteins with unique structures. They are integrated in the membranes of certain types of cells and have the function of converting the mechanical force exerted on the cells into electrical signals modifying the cellular activity. Piezo proteins, for example, act in bladder cells to detect when the bladder is full and in the cells lining the blood vessels to detect and help regulate changes in blood pressure. Piezo2 proteins act on the sensory nerve endings of the skin and joints, helping to mediate the senses of touch, pain and proprioception – the sense of limb disposition.
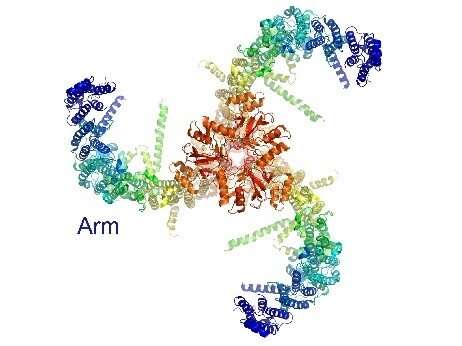
Triskelion architecture of Piezo1. Credit: Weill Cornell Medical College
Advances in imaging techniques have allowed scientists in recent years to determine the basic structure of Piezo1, a structure that Piezo2 is supposed to share primarily. From above, this structure has a three-armed, propeller or "triskelion" appearance.
On the side, it looks like a shallow bowl embedded in the cell membrane, with an ion channel at its center. The latter, when open, allows a flow of calcium and other positively charged ions into the cell.
The basic mechanism by which the mechanical force opens the ion channel has remained mysterious. But in the new study, Dr. Scheuring and Dr. MacKinnon and their colleagues, including the lead author, Dr. Yi-Chih Lin, postdoctoral associate in Anesthesiology, were able to get a clearer picture of how this works.
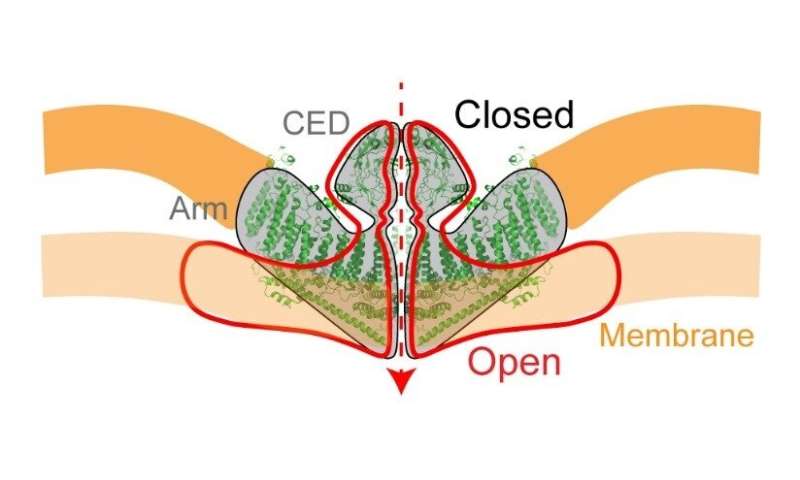
Side view of the piezo1. The red line indicates structural changes when the ion channel is open. Credit: Weill Cornell Medical College
They combined cryo-electron microscopy with a lesser known technique called high-speed atomic force microscopy, which produces an image of an object essentially by palpating its surface with the help of a mechanical probe. super sensitive. They showed with these methods that Piezo1 is an elastic structure that normally bends the cell membrane where it rests, but flattens out when a mechanical force is applied to the cell membrane, for example.
"As the membrane tension increases, the structure of the Piezo1 flattens and expands to occupy a larger area, which in turn opens the ion channel," said Dr. Scheuring.
He evoked the possibility that other stimuli stretching and flattening the piezoelectric structure, such as a pulling force exerted on his arms from the inside or on an external domain called CED from the outside. outside the cell, could in principle open the ion channel. a versatile mechanism adapted to the wide range of cell types and physiological functions in which it operates.
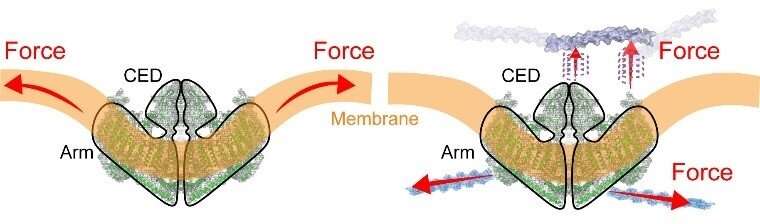
Mechanisms of action proposed by Piezo1 in response to the force. Left: Changes in membrane properties, such as tension or curvature, cause a force to open the piezo1. Right: The Piezo1 channel is activated when structures inside or outside the cell push or pull on the ion channel. Credit: Weill Cornell Medical College
In addition, given this wide range of cell types – in organs such as the lungs, bladder, intestines and pancreas, as well as in the blood vessels and the sensory nervous system – the discovery of the basic mechanism of the Piezoprotein protein could understand and treat many human diseases. To take one example, Dr. Scheuring said that if the membranes of the cells lining the blood vessels contain excess cholesterol, they would become stiffer, which would increase the background voltage of the integrated Piezo 1 proteins and potentially disrupt the ability normal of these proteins to detect and regulate blood pressure. .
"Our discovery leads to many predictions about the role of piezo proteins in the disease, which we and others can now study and study," he said.
The researchers are looking for the first time on the protein behind the sense of touch
Yi-Chih Lin et al, Force-induced conformational changes in PIEZO1, Nature (2019). DOI: 10.1038 / s41586-019-1499-2
Quote:
Scientists discover the basics of functioning of piezoelectric pressure-sensitive proteins (August 21, 2019)
recovered on August 21, 2019
at https://phys.org/news/2019-08-scientists-basics-pressure-sensing-piezo-proteins.html
This document is subject to copyright. Apart from any fair use for study or private research purposes, no
part may be reproduced without written permission. Content is provided for information only.
[ad_2]
Source link