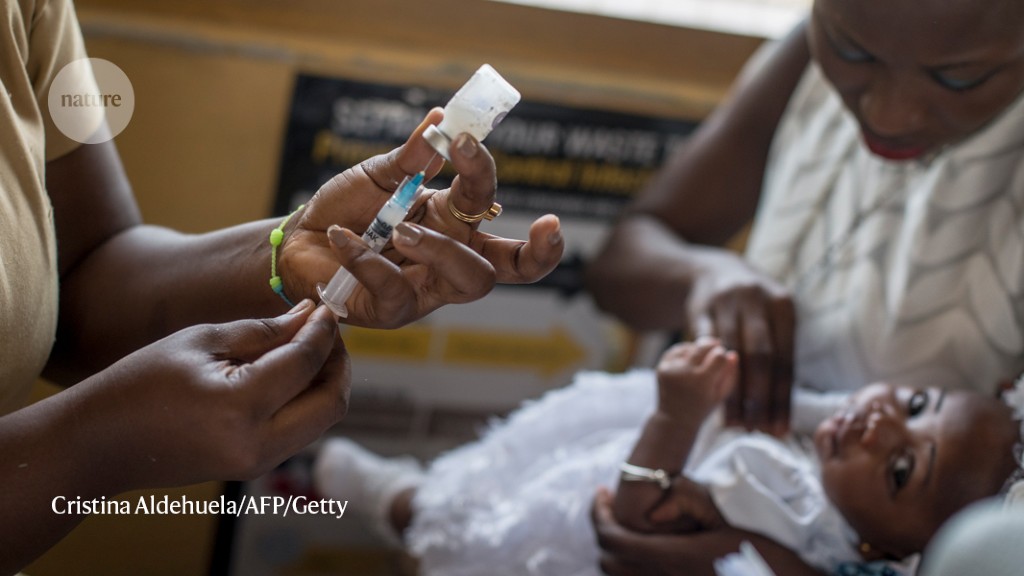
[ad_1]
More than 130 years after the appointment of the Plasmodium parasites behind malaria, the world now has its first vaccine approved against them. Many malaria researchers have celebrated the development, but others have expressed concerns about the deployment of a vaccine that is only moderately effective.
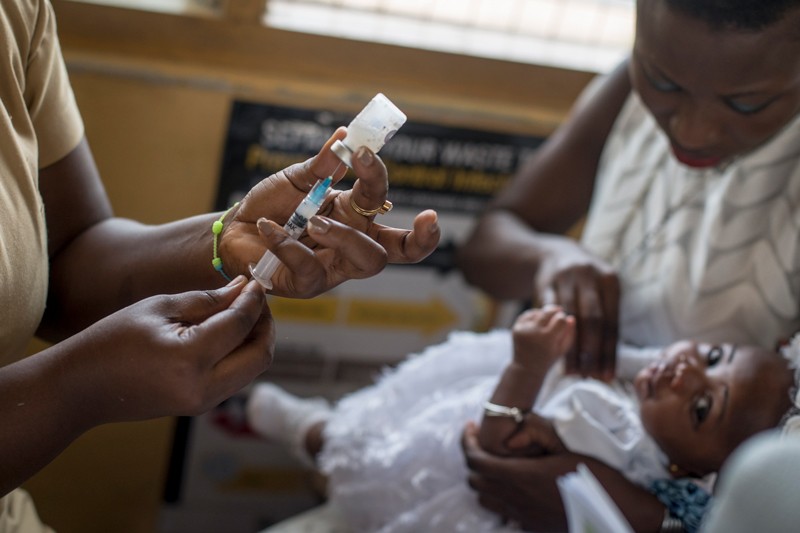
On October 6, the World Health Organization (WHO) endorsed the vaccine – called RTS, S – and recommended its widespread use in children in sub-Saharan Africa, home to the deadliest malaria parasite, Plasmodium falciparum.
“The RTS, S malaria vaccine – over 30 years of manufacture – is changing the course of public health history,” WHO Director-General Tedros Adhanom Ghebreyesus said at a briefing. press announcing approval.
Compared to other childhood vaccinations, RTS, S has only modest efficacy, preventing about 30% of severe malaria cases after a series of four injections in children under five.1.
Nonetheless, a modeling study suggests that it could prevent the deaths of 23,000 children per year, if the full series of doses were given to all children in countries with a high incidence of malaria.2 – making a significant dent in the enormous toll of the disease, which killed 411,000 people in 2018.
Leaders across Africa are now considering whether and how to deploy the vaccine. In Mali, for example, malaria researcher Alassane Dicko from the University of Bamako said Nature that shortly after the WHO announcement, the country’s health minister asked him what Mali needed to do to get the vaccine.
“I told him that we had to lobby as a country, at the highest levels of our government, to make this vaccine available at an affordable cost as soon as possible,” he says.
Three-decade effort
Researchers have been developing and testing the RTS, S vaccine – also known by its brand name, Mosquirix – since 1987, at a cost of more than $ 750 million. This was funded primarily by the Bill & Melinda Gates Foundation in Seattle, Washington, and the London-based pharmaceutical company GlaxoSmithKline (GSK).
Although clinical trials were completed in 2015, WHO subsequently recommended pilot studies to determine the feasibility and safety of this multidose vaccine outside of a clinical trial.
Gavi, the Vaccine Alliance, a health partnership based in Geneva, Switzerland, helped fund the pilot programs, which distributed 2.3 million doses of vaccine to Ghana, Kenya and Malawi. He reports that in these studies, hospitalizations for severe malaria were reduced by about 30%. These results have given WHO the confidence to recommend that four doses of the vaccine be given to children living in areas with moderate to high transmission of malaria.
However, Dicko says countries could achieve even greater declines in hospitalizations and deaths through tailor-made deployments.
In August, he and his colleagues published the results of a clinical trial finding that the RTS, S vaccine reduced childhood malaria deaths by 73% if children received three doses before the rainy season – when malaria peaks – and another dose before the rainy season the following two years3. Notably, this was done in conjunction with a method called seasonal malaria chemoprevention, in which healthy children take a monthly dose of antimalarial drugs to help prevent illness.
In addition to deciding how to deploy the vaccine, countries will need to determine how much it will cost to purchase and distribute it – and whether donors will help foot the bill.
Vaccine maker GSK has issued a statement committing to make 15 million doses available annually just above the cost of production. However, around 100 million doses will be needed each year for all children in high burden countries to receive the injections.2.
Could existing measures be eclipsed?
Some researchers fear that the enthusiasm for a vaccine overshadows existing malaria control measures that are already often underfunded, including insecticide programs and functioning health systems.
At a potential cost of around $ 5 per dose, the researchers suggest that deploying the vaccine, including its distribution, would cost around $ 325 million to administer each year in ten African countries with high malaria incidence. They point out that in some of these countries, other antimalarial measures have failed due to lack of support.
“I respect the researchers involved in this massive effort, but the reality is that so much money has been invested in this vaccine, even when the results of the studies are disappointing,” says Badara Cissé, malaria researcher at the Institut de health research, epidemiological surveillance and training in Dakar. “I don’t think a 30% effective vaccine would be acceptable to Americans.”
Still, he and James Tibenderana, a Ugandan epidemiologist at the Malaria Consortium in London, say the RTS, S vaccine could have an impact in some areas. To achieve this, Tibenderana stresses the need for extensive communication campaigns, so that disinformation does not hinder deployment.
“People will wonder why a 30-year-old, partially effective vaccine is suddenly introduced during a pandemic – and only targets Africans,” he says. “The misinformation around COVID-19 vaccines should teach us that we cannot take community trust for granted. “
Despite the long road ahead, he and others are grateful for the WHO decision. “With the devastation of COVID-19, and with the stalled progress in the fight against malaria, and the news of antimalarial drug resistance, it is edifying to see some positive news,” he said.
[ad_2]
Source link