[ad_1]
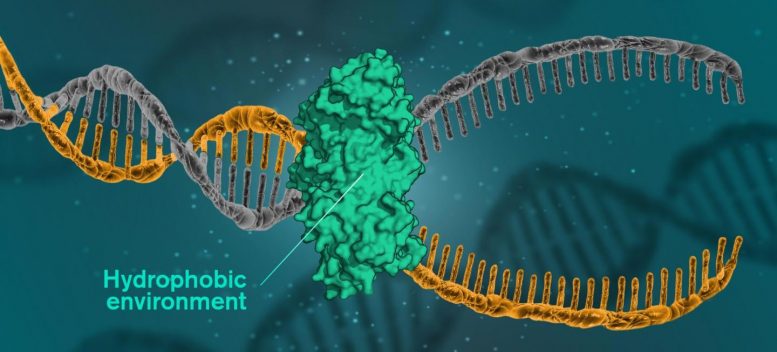
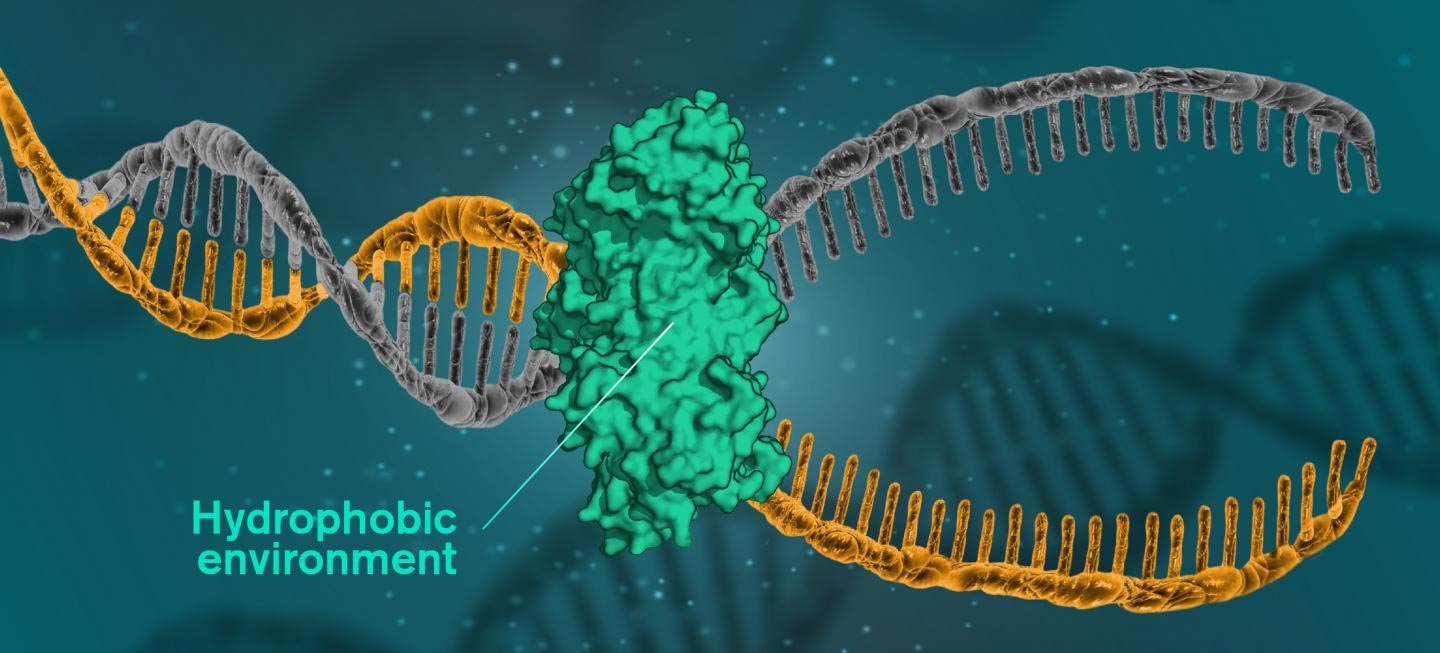
For DNA to be read, replicated or repaired, the DNA molecules must open themselves. This occurs when cells use a catalytic protein to create a hydrophobic environment around the molecule. Illustration Credit: Yen Strandqvist / Chalmers University of Technology
Researchers at the Chalmers University of Technology in Sweden refute the prevailing theory of DNA links with. As is generally believed, it is not hydrogen bonds that bind the two sides of the DNA structure together. Instead, water is the key. This discovery paves the way for new research knowledge in medicine and life sciences. The results of the researchers are presented in the journal PNAS.
DNA consists of two strands, composed of sugar molecules and phosphate groups. Between these two strands are nitrogenous bases, the compounds that make up the genes of organisms, with hydrogen bonds between them. Until now, it was commonly accepted that these hydrogen bonds maintained the cohesion of the two strands.
But now, researchers at the Chalmers University of Technology show that the secret of the helical structure of DNA may lie in the fact that the molecules have a hydrophobic interior, in an environment consisting mainly of water. The environment is therefore hydrophilic, while the nitrogenous bases of the DNA molecules are hydrophobic, repelling the surrounding waters. When the hydrophobic units are in a hydrophilic environment, they group together to minimize their exposure to water.
The role of hydrogen bonds, previously considered crucial for the consolidation of DNA helices, seems to be more related to the sorting of base pairs, so that they bind in the right order.
The discovery is crucial to understand the relationship between DNA and its environment.

Bobo Feng, postdoc, chemistry and chemical engineering, Chalmers University of Technology. Credit: Johan Bodell / Chalmers University of Technology
"Cells want to protect their DNA and not expose it to hydrophobic environments, which can sometimes contain harmful molecules," said Bobo Feng, one of the researchers at the origin of the 39; study. "But at the same time, the DNA cells must open to be able to be used."
"We think that the cell retains its DNA in an aqueous solution most of the time, but as soon as a cell wants to do something with its DNA, like reading it, copying it or repairing it, it exposes it." DNA to a hydrophobic environment. "
Reproduction, for example, implies that the base pairs dissolve and open. The enzymes then copy both sides of the helix to create a new DNA. When it is a question of repairing the damaged DNA, the damaged areas are subjected to a hydrophobic environment, to be replaced. A catalytic protein creates the hydrophobic environment. This type of protein is at the heart of all DNA repairs, which means that it could be the key to fighting many serious diseases.
Understanding these proteins could provide a lot of new information on how we could, for example, fight resistant bacteria or even potentially cure cancer. Bacteria use a protein called RecA to repair their DNA, and researchers believe their findings could provide new insights into how this process works – potentially offering methods to stop it and kill the bacteria.
In human cells, the Rad51 protein repairs the DNA and fixes the mutated DNA sequences, which might otherwise cause cancer.
"To understand cancer, we need to understand how DNA is repaired. To understand this, we must first understand the DNA itself, "says Bobo Feng. "Until now, we have not done it because we thought hydrogen bonds were what held him. We have now shown that it is rather hydrophobic forces. We have also shown that DNA behaves totally differently in a hydrophobic environment. This could help us understand the DNA and its repair. Nobody had previously placed DNA in a hydrophobic environment like this one and studied its behavior. No wonder no one has discovered it so far.
More information on methods used by researchers to show how DNA binds:
The researchers studied the behavior of DNA in a more hydrophobic environment than the normal method with which they were the first to experiment.
They used the hydrophobic solution of polyethylene glycol and, step by step, modified the environment of the DNA, which went from the naturally hydrophilic environment to a hydrophobic environment. They wanted to know if there is a limit to which DNA begins to lose its structure, while DNA has no reason to bind because the environment is no longer hydrophilic. The researchers observed that when the solution reached the boundary between hydrophilic and hydrophobic, the spiral shape characteristic of the DNA molecules began to break down.
After a closer inspection, they found that when the base pairs separated from each other (due to external influences or simply random movements), holes formed in the structure, which allowed the Water to penetrate to the interior. Because DNA wants to keep his interior dry, he presses together, with the base pairs coming together again to bring out the water. In a hydrophobic environment, this water is missing, so the holes remain in place.
Reference: "Hydrophobic catalysis and the potential biological role of DNA de-stacking induced by environmental effects" by
[ad_2]
Source link