[ad_1]
Scientists have found a new way of future explorers of Mars could potentially generate their own oxygen.
March It's far from the Earth. Therefore, creating breathable air on site would save money and effort in the transportation of oxygen on our planet.
A research team discovered this new oxygen-generating reaction while studying comets. Most of these small icy worlds originate in a remote area of the solar system called the Oort Cloud, well beyond the orbit of Neptune. If the orbit of a comet brings it closer to the sun, the heat begins to push the cometary ice into the space. This reaction produces long tails that can extend over thousands of kilometers.
Related: 7 biggest mysteries of Mars
A team of researchers at California's Institute of Technology (Caltech) in Pasadena has found a new way to explain how comets generate molecular oxygen, the two oxygen atoms that combine to form a breathable air.
An already known method uses kinetic energy. A sublimated comet is a busy environment, where the solar wind (the constant stream of particles emanating from the sun) can push floating water molecules into the surface of the comet at high speed. If there are compounds containing oxygen on the surface, fairing water molecules can tear oxygen atoms and produce molecular oxygen.
The team discovered that molecular oxygen can also be produced through carbon dioxide reactions. (Carbon dioxide contains a single carbon atom and two oxygen atoms.) Caltech's former postdoctoral fellow, Yunxi Yao, and Caltech's chemical engineering professor, Konstantinos Giapis, have simulated this reaction. by introducing carbon dioxide into a gold foil. Since the gold leaf can not be oxidized, it should not produce molecular oxygen. But when carbon dioxide enters the sheet at high speed, the gold surface emits molecular oxygen.
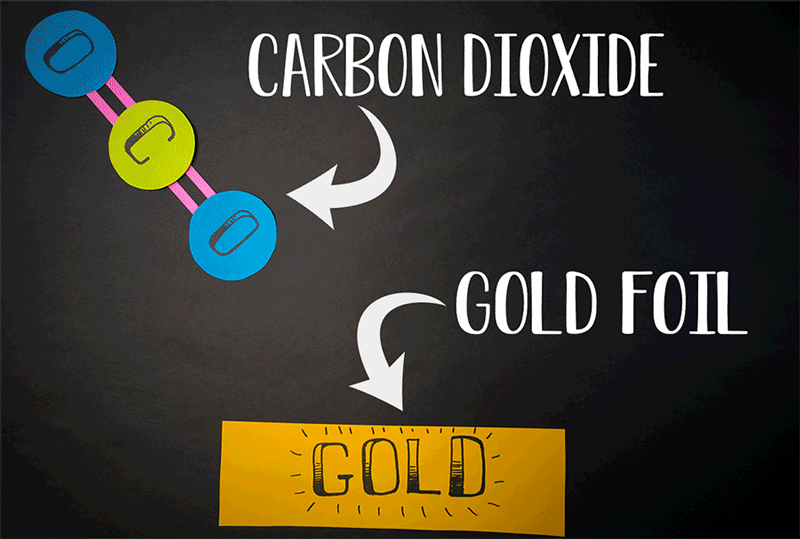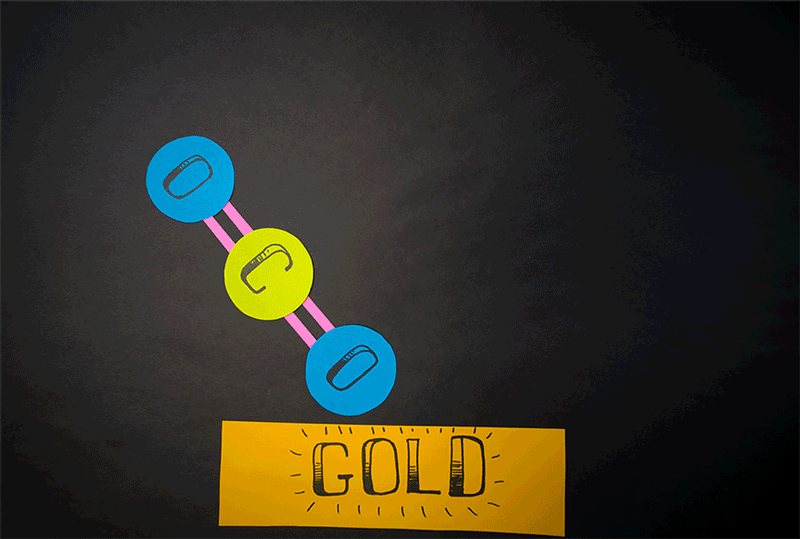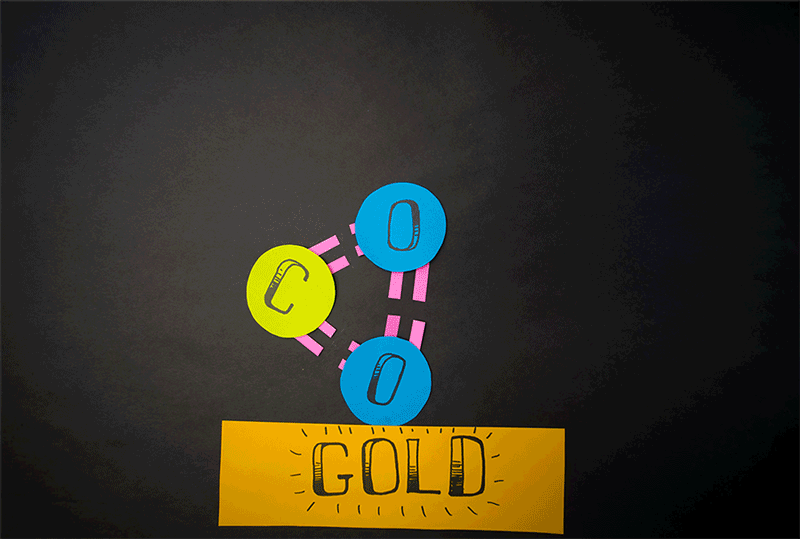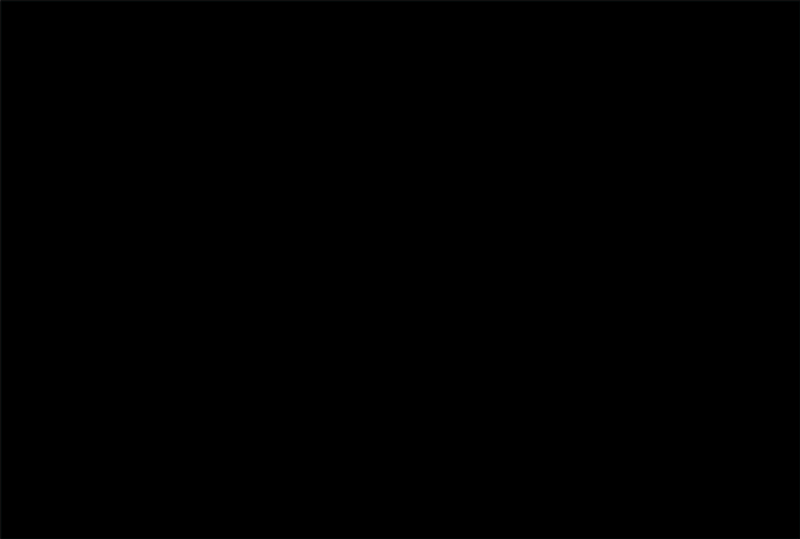
Diagram showing how carbon dioxide can be converted to molecular oxygen inside a reactor.
(Image: © Caltech)
"It meant that the two atoms of oxygen came from the same CO2 [carbon dioxide] molecule, dividing it effectively in an extraordinary way ", representatives of Caltech said in a statement.
To better understand how carbon dioxide can degrade in molecular oxygen, Caltech chemistry professor Tom Miller and postdoctoral fellow Philip Shushkov created a computer simulation.
A challenge in modeling the reaction is that the molecules that react are very "excited", which means they vibrate and spin in a complex way, the researchers said.
"In general, excited molecules can lead to unusual chemistry, so we started with that," said Miller in his statement. "But, to our surprise, the excited state did not create molecular oxygen – instead, the molecule was broken down into other products."
Rather, scientists have discovered that extremely "curved" carbon dioxide molecules – those with unusual geometry – can be created without exciting carbon dioxide. This would produce oxygen.
When Yao and Giapis broke the carbon dioxide molecules in a gold foil, they electrically charged the individual molecules of carbon dioxide and then accelerated them with the help of an electric field. . However, Giapis said the reaction could also occur at a slower speed, which could explain why there is oxygen floating high up in the sky. Martian atmosphere.
"You can throw a rock with enough speed with CO2 [carbon dioxide] and achieve the same thing, "he said in a statement. It would have to be moving about as fast as a comet or asteroid moves in space. "
Related: How to live on Mars could challenge settlers
Previously, scientists thought that the minute concentration of atmospheric oxygen in Mars is probably generated after the sun's ultraviolet light hits the carbon dioxide molecules in the air of the red planet. Giapis believes, however, that Martian oxygen could also be generated when dust particles, accelerated at a very high speed in the atmosphere, break in carbon dioxide molecules.
The reactor used by Giapis is at very low efficiency, generating only one or two molecules of oxygen per 100 molecules of carbon dioxide passing through the accelerator. Giapis, however, said his reactor could perhaps be modified someday to create breathable air for astronauts on Mars. And on Earth, the reactor could be useful for extracting carbon dioxide (which is also a powerful greenhouse gas and the main factor in global warming) out of the atmosphere and convert it to oxygen.
"Is it an end device? No. Is it a device that can solve the problem with Mars? No," he says. "But it's a device that can do something very difficult – we're doing crazy things with this reactor."
An article based on the research, directed by Yao, was published last week in the newspaper Nature Communications.
By the way, NASA is about to test the technology that generates oxygen on Mars. A technological demonstrator called MOXIE (Experience using in situ resources on Mars oxygen) will fly aboard the agency 's 2020 rover, which is expected to be launched next summer and landed on the red planet in February 2021. MOXIE will electrochemically divide atmospheric carbon dioxide This method could be expanded to help people living on the red planet.
Follow Elizabeth Howell on Twitter @howellspace. follow us on Twitter @Spacedotcom and on Facebook.
[ad_2]
Source link