[ad_1]
The FDA said in a statement that it was the second approved cancer treatment based on a tumor biomarker instead of the place in the body where the tumor originated.
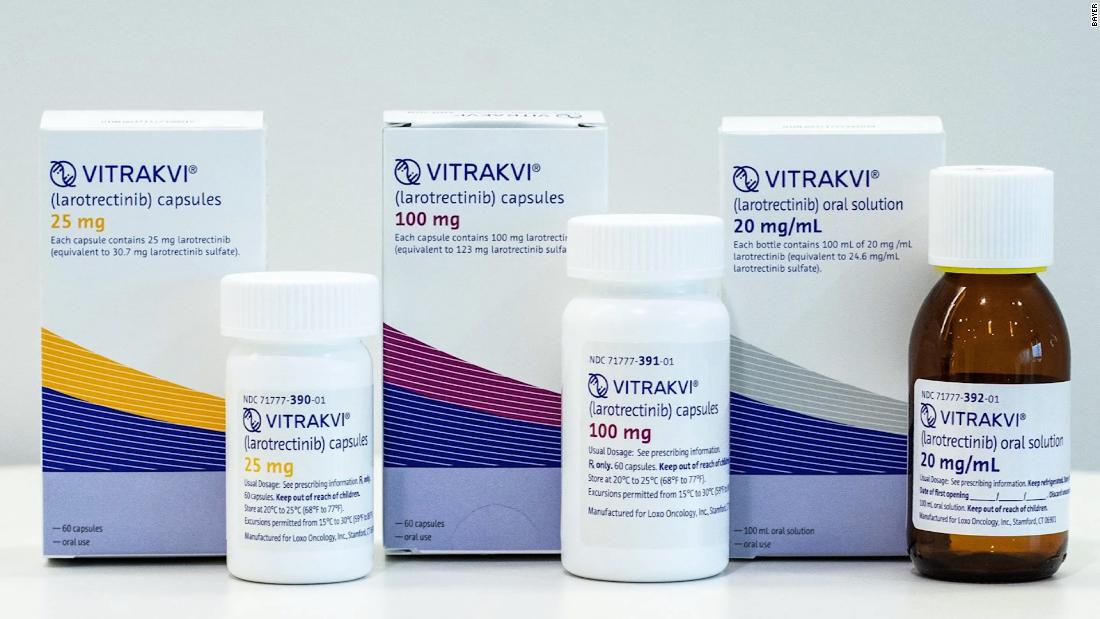
Vitrakvi will be used for the treatment of solid tumors with NTRK gene fusion (neurotrophic receptor tyrosine kinase) that do not have a known resistance mutation, that are not metastatic or whose surgical ablation is likely to lead to severe morbidity. no alternative treatments or have progressed after the treatments.
NTRK genes are rare but occur in many types of cancer, the FDA said, such as secretory breast carcinoma and infantile fibrosarcoma.
In addition to the tablets, the agency has also approved a diagnosis to detect the mutation.
"About 25 to 30% of AML patients have FLT3 mutations, which are associated with a particularly aggressive form of the disease and a higher risk of relapse," said Dr. Richard Pazdur, Center Director. oncology of the FDA. Excellence, said in the statement.
AML is a rapidly evolving cancer that affects the number of normal blood cells and requires ongoing transfusions, the FDA said.
Both treatments received the "priority review" designation.
"A priority assessment designation will direct overall attention and resources on the assessment of drug claims that, if approved, would constitute significant improvements in the safety or efficacy of the drug." 39, effectiveness of treatment, diagnosis or prevention of serious conditions compared to standard applications, "says the FDA.
Source link