
[ad_1]
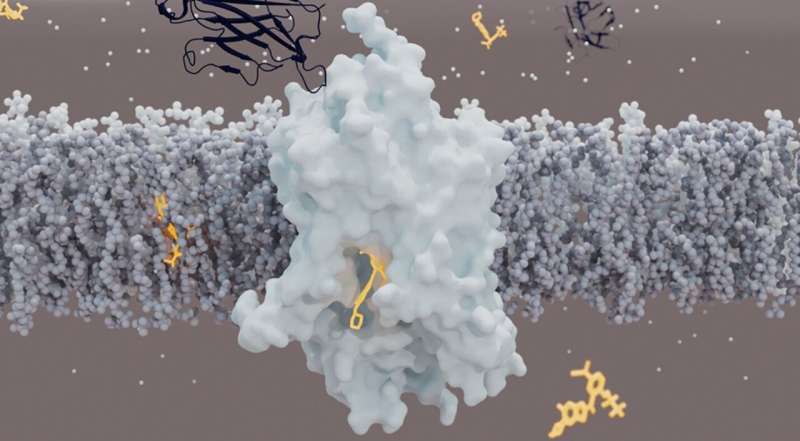
Credit: GlyT1 (light blue) is a protein that transports glycine across the cell membrane (gray). To do this, it opens alternately to the outside and the inside of the cell. Unlike other neurotransmitter transporters, it is bound by its inhibitor (orange) on the intracellular side rather than the extracellular one. Sybody, a synthetic mini-antibody (dark blue), also inhibits GlyT1 by binding to a new extracellular site. Credit: Azadeh Shahsavar / DANDRITE
Glycine is the smallest amino acid, one of the building blocks of protein. It also acts as a neurotransmitter in the brain, allowing neurons to communicate with each other and modulate neural activity. Many researchers have focused on increasing glycine levels in synapses to find an effective treatment for schizophrenia. This could be done by using inhibitors targeting the glycine transporter 1 (GlyT1), a protein that is found in the membranes of neuronal cells and is responsible for the uptake of glycine into neurons. However, the development of these drugs was hampered because the 3D structure of GlyT1 was not known.
To determine the structure of GlyT1, researchers from the Danish Translational Neuroscience Research Institute (DANDRITE), which is part of the EMBL Nordic Partnership for Molecular Medicine, F. Hoffmann-La Roche, EMBL Hamburg, University of Zurich , Aarhus University, and Linkster Therapeutics have joined forces. “This project required multidisciplinary collaboration and unique expertise from different laboratories over several years,” says Azadeh Shahsavar, first author of the study and now assistant professor at DANDRITE. She performed the measurements for the study during her time as a post-doctoral fellow in the EMBL Interdisciplinary Postdocs (EIPOD) program, during which she worked at EMBL Hamburg, DANDRITE and Roche.
Poul Nissen, Director of DANDRITE and Principal Investigator of the study, comments: “We are extremely grateful to the EMBL EIPOD program and the EMBL Nordic Partnership for keeping us on track for so long and allowing us to explore very difficult approaches. not succeeded without it, and without Azadeh’s perseverance of course! “
Overcome challenges by studying the glycine transporter 1
GlyT1 has been shown to be particularly difficult to study because it is unstable when extracted from the cell membrane. To stabilize it, scientists have combined several approaches, such as creating more stable variants of the protein. To catch the transporter in a clinically relevant state, the team used a chemical created by Roche that binds and stabilizes GlyT1 from the inside, and engineered a synthetic mini-antibody (sybody) that binds it from the outside. .
Scientists tested 960 different conditions and managed to get GlyT1 crystals in one of them. “The crystals were very small and difficult to image. We chose to measure them on EMBL Hamburg’s P14 beamline, which is well suited for difficult experiments like this,” says Azadeh. The X-ray beam at P14 is particularly powerful and focused, and its equipment has characteristics suitable for working with even micrometric-sized crystals. However, the quality of the crystals was variable, which made data collection difficult. In the end, Azadeh’s persistence paid off. “I remember when I saw the electron density of the inhibitor for the first time. I was so excited I couldn’t sleep for two nights, ”she says. “You live for these rewarding moments.”
The last challenge was data analysis. While the crystals gave only weak diffraction patterns due to their small size, the strong x-rays destroyed the crystals in less than a second. A single crystal would only give partial information about the structure, so Azadeh had to collect data from hundreds of crystals. “The processing of such a large amount of data was made possible by the unique infrastructure of EMBL Hamburg,” she says. Combining partial datasets was complex for the existing software, but the Schneider group at EMBL Hamburg wrote software specifically designed for such cases. This allowed Azadeh to merge datasets into a complete image of GlyT1 at a resolution of 3.4 Å (1 Å, or ångström, is one ten billionth of a meter – about the size of a typical atom). “I really enjoyed working with people from different scientific backgrounds. Each brought their unique expertise that made this study possible, ”says Azadeh.
For Thomas Schneider, co-head of research infrastructures at EMBL Hamburg, the study is a perfect example underlining the importance of both scientific excellence and the availability of cutting-edge infrastructure to advance research. research. “For ambitious projects like this, we are happy to leverage the methodological expertise of our staff and make full use of the technological capabilities of our beamlines and sample preparation facilities. The high-intensity microfocused beam produced by the PETRA III synchrotron on the DESY campus and the versatile high-precision diffractometer that was developed in a collaboration between EMBL Hamburg, EMBL Grenoble and ARINAX were essential for this project. “
Azadeh agrees. “The excellence, infrastructure, hardware and software provided by EMBL are of the highest quality and are constantly being improved,” she adds.
Blueprint for new therapies
Analysis revealed an unexpected structure of GlyT1. Unlike other neurotransmitter transporters, which are bound by their inhibitors on the outer side of the cell membrane, GlyT1 is bound by its inhibitor on the inner side. “The structure came as a surprise to us. It seems that the GlyT1 inhibitor must first cross cell membranes before it can access GlyT1 from inside neurons,” says Roger Dawson, lead author of the study. .
“This structure provides a model for the development of new inhibitors of GlyT1, whether organic molecules or antibodies,” explains Roger. “The sybody developed for this study binds GlyT1 to a previously unknown binding site and locks it in a state where it can no longer transport glycine. We could use this knowledge to develop drugs that target not only GlyT1, but also other membrane transport proteins in the future. ”
Determination of glycine transporter opens new avenues in psychiatric drug development
Azadeh Shahsavar et al, structural insights on glycine reuptake inhibition, Nature (2021). DOI: 10.1038 / s41586-021-03274-z
Provided by the European Molecular Biology Laboratory
Quote: Structural Biology Opens New Perspectives for the Treatment of Psychiatric Disorders (2021, April 6) Retrieved April 7, 2021 from https://phys.org/news/2021-04-biology-perspectives-psychiatric-disorders.html
This document is subject to copyright. Other than fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.
[ad_2]
Source link