[ad_1]
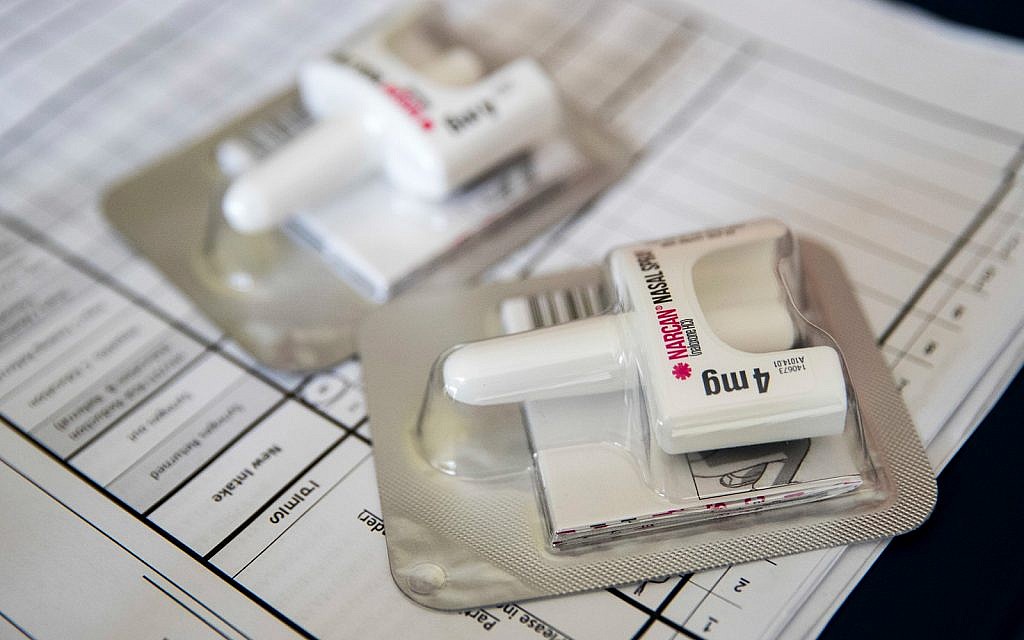
US and US regulators have approved the first generic version of Narcan Nasal Spray, a drug that reverses opioid overdoses.
The Food and Drug Administration on Friday approved the naloxone spray from Teva Pharmaceuticals in Israel.
Naloxone has been sold as a nasal spray in the United States since 2016 under the Narcan brand. Pharmacists can dispense it without a prescription. It is also sold as a generic or branded drug in the form of automatic injectors, pre-filled syringes and vials.
Receive by email the Start-Up Daily Israel Start-Up and never miss our best stories
Free registration
A pack of two nasal sprays Narcan costs about $ 130 to $ 150 without insurance. Teva has not immediately communicated the price of the product or the moment it will be available; its offices were closed Friday.
More than 47,600 Americans have died from an opioid overdose in 2017, a steadily growing record for two decades.
[ad_2]
Source link