
[ad_1]

A new study from the lab of Whitehead Institute member Yukiko Yamashita suggests that the chromosomal organizing system made possible by satellite DNA is one reason why organisms of different species cannot produce viable offspring. Credit: Jennifer Cook-Chrysos / Whitehead Institute
More than 10 percent of our genome is made up of repetitive and seemingly insane stretches of genetic material called satellite DNAs that don’t encode any protein. In the past, some scientists have called this DNA “genomic waste”.
In a series of articles spanning several years, however, Yukiko Yamashita, a member of the Whitehead Institute, and her colleagues argued that satellite DNA is not unwanted, but rather plays a vital role in the cell: it works with cellular proteins for individual chromosomes united in a single nucleus.
Now, in the latest installment of their work, published online July 24 in the journal Molecular biology and evolution, Yamashita and former postdoctoral fellow Madhav Jagannathan, currently an assistant professor at ETH Zurich, Switzerland, take these studies a step further, proposing that the chromosome organization system made possible by satellite DNA is the one of the reasons why organisms of different species cannot produce viable offspring. .
“Seven or eight years ago when we decided we wanted to study satellite DNA, we had no plan to study evolution,” said Yamashita, who is also a professor of biology at the Massachusetts Institute of Technology. and researcher at the Howard Hughes Medical Institute. . “It’s a great fun part of science – when you don’t have a preconceived idea and just follow suit until you stumble upon something completely unexpected. “
The origin of species: DNA edition
Researchers have known for years that satellite DNA is highly variable between species. “If you look at the chimpanzee genome and the human genome, the protein coding regions are 98%, 99% identical,” she says. “But the part of unwanted DNA is very, very different.”
“These are the fastest growing sequences in the genome, but the earlier point of view was, ‘Well these are unwanted sequences, who cares if your junk is different from mine?” Jagannathan said. .
But as they investigated the importance of satellite DNA to the fertility and survival of pure species, Yamashita and Jagannathan got their first clue that these repetitive sequences might play a role in speciation.
When researchers removed a protein called Prod that binds to a satellite DNA sequence specific to the fruit fly Drosophila melanogaster, the fly’s chromosomes scattered outside the nucleus in tiny balls of cellular material called micronuclei. , and the flies died. “But we realized at this point that this [piece of] The satellite DNA that was bound by the Prod protein was completely absent in the closest relatives of Drosophila melanogaster, ”said Jagannathan. “It absolutely does not exist. So it’s an interesting little problem. “
If this piece of satellite DNA was essential for the survival of one species but was lacking in another, it could imply that the two species of flies had developed different satellite DNA sequences for the same role over time. And since satellite DNA played a role in keeping all chromosomes together, Yamashita and Jagannathan wondered if these evolved differences could be one of the reasons different species are reproductively incompatible.
“After performing the function [of satellite DNA in the cell], the fact that the satellite DNA is quite different between species really struck like lightning, “Yamashita said.” All of a sudden it became a whole different investigation. ”
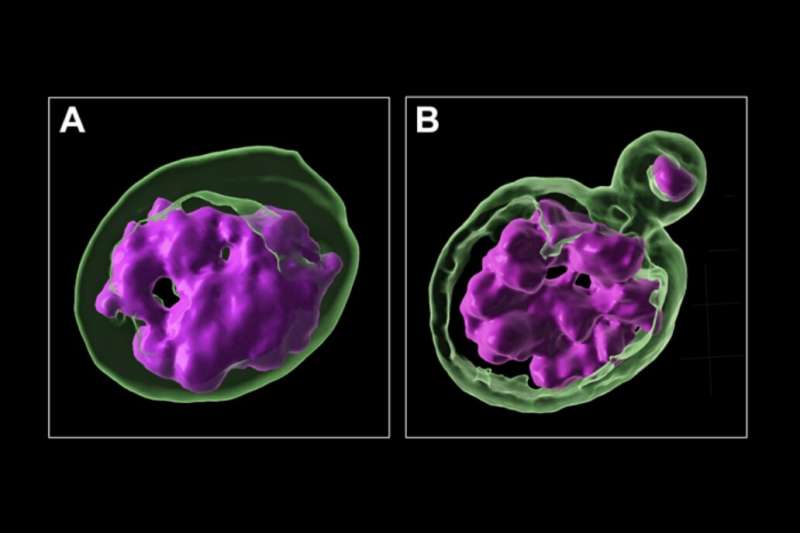
3D rendering of a cell with an intact nucleus (A) and a cell with a micronucleus (B). DNA is marked in magenta and the nuclear envelope is marked in green. Credit: Franziska Brändle
The story of two species of fruit flies
To study how differences in satellite DNA might underlie reproductive incompatibility, the researchers decided to focus on two branches of the fruit fly family tree: the classic laboratory model Drosophila melanogaster, and its closer parent, Drosophila simulans. These two species diverged from each other two to three million years ago.
Researchers can breed a female Drosophila melanogaster with a male Drosophila simulans, “but [the cross] generates very unhappy offspring, ”Yamashita said. “Either they are sterile or they die. “
Yamashita and Jagannathan bred the flies together, then studied the tissues of the offspring to see what made these “unfortunate” hybrids to drop like flies. They noticed something interesting right away: “When we looked at these hybrid tissues, it was very clear that their phenotype was exactly the same as if you had disrupted the satellite DNA. [-mediated chromosomal organization] of a pure species, ”Yamashita said. “The chromosomes were scattered and not encapsulated in a single nucleus. “
In addition, researchers could create a healthy hybrid fly by mutating certain genes in mother flies called “hybrid incompatibility genes,” which were found to localize on satellite DNA in cells of pure species. Through these experiments, researchers were able to demonstrate how these genes affect chromosomal packaging in hybrids and identify for the first time the cellular phenotypes associated with them. “I think for me that’s probably the most critical part of this article,” Jagannathan said.
Taken together, these results suggest that because satellite DNA mutates relatively frequently, the proteins that bind satellite DNA and hold chromosomes together must evolve to keep up, leading each species to develop its own “strategy” for working with it. Satellite DNA. When two organisms with different strategies cross each other, a clash occurs, causing the chromosomes to disperse outside the nucleus.
In future studies, Yamashita and Jagannathan hope to put their model to the ultimate test: if they can design a protein capable of binding satellite DNA from two different species and holding chromosomes together, they could theoretically “save” one. doomed hybrid, allowing to survive and produce viable offspring.
This feat of bioengineering is probably years away. “At the moment, it’s just a purely conceptual thing,” Yamashita said. “In doing this DIY, there are probably a lot of details that will need to be worked out.”
For now, the researchers plan to continue studying the roles of satellite DNA in the cell, armed with their new knowledge about the role it plays in speciation. “For me, the surprising part of this article is that our assumption was correct,” Jagannathan said. “I mean, in retrospect, there are so many ways that things could have been at odds with what we assumed, so it’s pretty amazing that we kind of were able to chart a clear course from start to finish.”
Scientists discover role for “junk” DNA
Madhav Jagannathan et al, Defective Satellite DNA Clustering in Chromocenters Underlies Hybrid Incompatibility in Drosophila, Molecular biology and evolution (2021). DOI: 10.1093 / molbev / msab221
Madhav Jagannathan et al, Comparative analysis of satellite DNA in the Drosophila melanogaster species complex, G3 Genes | Genomes | Genetics (2017). DOI: 10.1534 / g3.116.035352
Madhav Jagannathan et al, A conserved function for pericentromeric satellite DNA, eLife (2018). DOI: 10.7554 / eLife.34122
Madhav Jagannathan et al, The modular mechanism of chromocenter formation in Drosophila, eLife (2019). DOI: 10.7554 / eLife.43938
Provided by Whitehead Institute for Biomedical Research
Quote: So-called “junk” DNA plays a key role in speciation (2021, 23 Aug) retrieved 23 Aug 2021 from https://phys.org/news/2021-08-so-posed-junk-dna -key-role .html
This document is subject to copyright. Other than fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.
[ad_2]
Source link