
[ad_1]
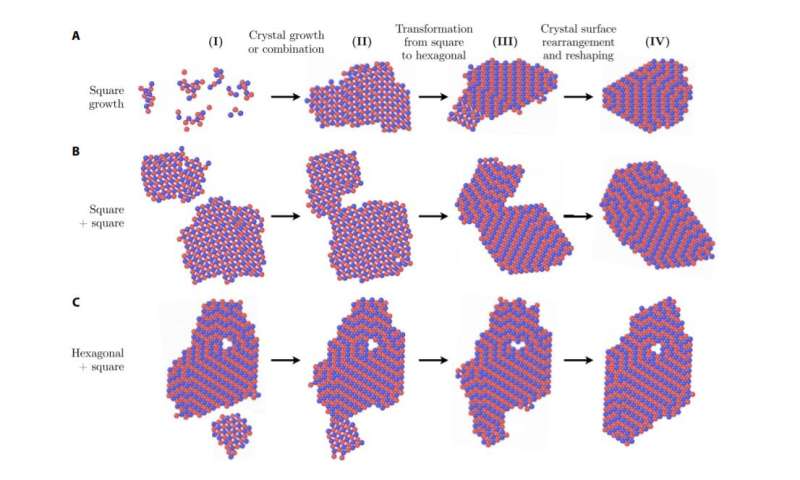
Snapshots of MD simulations of self-assembly with EAA / EAB interaction forces = EBB / EAB = 0.15, kBT / EAB temperature = 0.1 and density rs2 = 0.1. Examples of square transformations to hexagonal due to the growth (A) of a square cluster, (B) the attachment of a square cluster to another and (C) the attachment of a square cluster to a hexagonal cluster. Credit: Evan Pretti, Hasan Zerze, Song Minseok, Yajun Ding, Runfang Mao, Jeetain Mittal
The technology is getting smaller and smaller, which is good news.
The ability to make materials with optical, electrical, and mechanical properties from very small particles could have far-reaching applications. For example, microparticles grafted with DNA can be used in medicine to improve detection, imaging, and treatment administration. A better understanding of the behavior of these materials could help fulfill the promise of precision medicine, among other applications.
Much remains to be learned about the best way to lead the manufacture of these micromaterials. The process of self-assembly of micro-particles functionalized by DNA leads to a crystallization, that is to say to the transformation of atoms and molecules into a highly structured form. called crystal. Crystallization begins with nucleation – the process by which atoms or molecules cluster on a microscopic scale. If the clusters become sufficiently stable and sufficiently large, crystals can develop. Atoms and compounds can generally form more than one crystalline structure, called polymorphism. The arrangement of the particles is determined during the first stages of crystallization.
According to Jeetain Mittal, professor of chemical and biomolecular engineering at the University of Lehigh, structural transformations involving polymorphic potential during crystallization have been classically attributed to kinetic effects, or to the rate of nucleation, allowing predict which structures can be observed when crystals form. This is in line with the classical theory of nucleation.
Now, Mittal and his team have shown that kinetic effects may not be able to fully explain structural transformation in all polymorphic situations and that surface thermodynamics – related to crystallite size versus velocity – can to be critical for transformations between crystalline structures. The team has found a new path for the structural transformation of a square network to a hexagonal network during crystal growth, which is dictated by thermodynamics.
According to many previous systems, according to Mittal, crystallites with a structural polymorphism have been attributed to kinetic effects, related to the nucleation rate. In their work, Mittal and his collaborators provide a solid computation to demonstrate that the structural transformation can be entirely thermodynamic, contrary to the kinetic argument, from theoretical and computational points of view. In addition, a similar structural transformation is observed in a more detailed modeled system using a coarse grain model representing a DNA functionalized particle. This clearly shows that such structural transformations can be much more general and can be related to more realistic systems.
"Understanding the crystallization process is particularly important for controlling and predicting the structure produced," says Runfang Mao, currently a PhD student at Lehigh. student and co-author on paper. "Although it is useful in many cases, the classical theory of nucleation is considered invalid in many systems, we show that this size-dependent structural transformation is one of these exceptions and is motivated by By the thermodynamic properties of crystallites of finite size, such a size-dependent structural transformation has not been clearly illustrated elsewhere in the literature ".
Their conclusions were published today in Progress of science in the article "Size-dependent thermodynamic structural selection in colloidal crystallization". In addition to Mittal and Mao, the authors include Evan Pretti, Hasan Zerze, Minseok Song and Yajun Ding, all present and former students of the P. de Lehigh Chair. Rossin College of Engineering and Applied Science.
Mittal and his team have studied how particular mixtures of colloids and strands of DNA attached to their surfaces crystallize in two-dimensional networks when the colloids interact with each other. As Pretti explains, crystallization starts from small clusters of growing and aggregating particles, and under certain conditions these crystallites can start in one crystalline structure and transform into another over time. .
"We find that, for our system, these transformations can be explained in terms of how the relative thermodynamic stabilities of different structures are affected by the size of the crystallites," says Pretti. "In particular, for quite small crystallites, the thermodynamics of the surfaces becomes large enough to influence the structure, which triggers the transformations observed during the self-assembly."
According to Mittal, these DNA functionalized systems are of particular interest in the field of colloidal assembly, because of the great flexibility and variety of possibilities offered by the use of different types of particles and DNA sequences. Their results, however, are not limited to such systems, but could lead to a better understanding of how other types of crystallization processes work and can be controlled.
The team began using standard molecular dynamics simulations to understand the behavior of their system. To prove that the transformations observed were of thermodynamic origin, they used an existing method to calculate the relative thermodynamic stabilities of periodic crystalline solids and modified them to analyze their finite size crystallites.
"We have identified a structural transformation that is reversible and that can be explained using only the thermodynamics of finite-sized crystals themselves," explains Mittal. "Our work may provide a new way to examine and explain the transformations of particle systems functionalized by DNA and potentially other types of crystals as well."
Determination of the crystal structure of a DNA stabilized silver nanocluster
"Size-dependent thermodynamic structural selection in colloidal crystallization" Progress of science (2019). advance.sciencemag.org/content/5/9/eaaw5912
Quote:
The team discovers that polymorphic selection during crystal growth may be dictated by thermodynamics (13 September 2019)
recovered on September 14, 2019
at https://phys.org/news/2019-09-team-polymorph-crystal-growth-thermodynamically.html
This document is subject to copyright. Apart from any fair use for study or private research purposes, no
part may be reproduced without written permission. Content is provided for information only.
[ad_2]
Source link