[ad_1]
THE government responded after the release of the vaccine manufactured in the North East, Novavax faced significant delays and may not be ready until next year at the earliest.
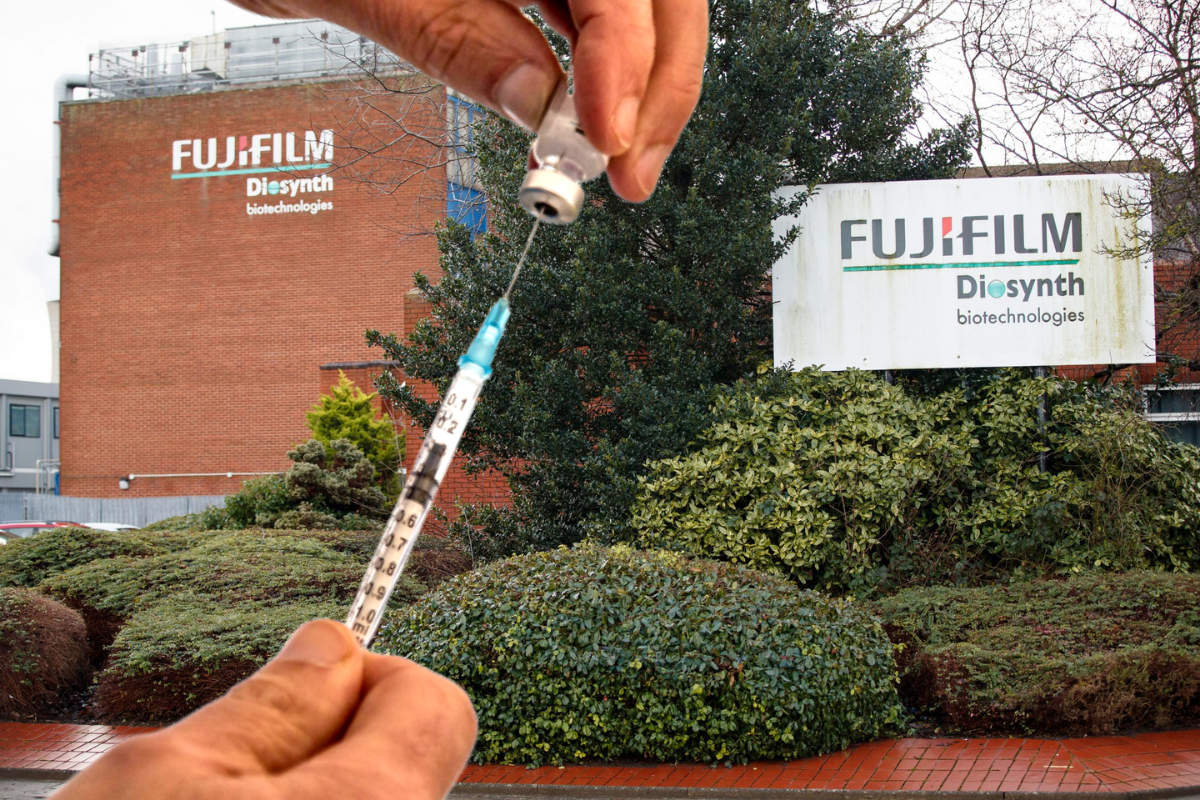
The Northern Echo revealed how the Novavax vaccine, which if successful would be manufactured by Fujifilm Diosynth in Billingham, has yet to be submitted to UK regulators for approval.
It came as participants in a trial in the Northeast said they had been “ditched” after learning that advice had yet to be released on the safety of having an alternate jab.
EXCLUSIVE: Covid vaccine manufactured in northeast faces delays – and may not be ready until “NEXT YEAR”
Those who took part in a trial at Hartlepool University Hospital over the past twelve months said they now feared being left unprotected during the winter.
Meanwhile, they said they found themselves at a serious disadvantage as they cannot travel overseas as their jab is not recognized by other countries.

A source close to the Novavax study said that with the third phase of the trials underway several months ago, there was a common understanding that the vaccine would be approved in “a few weeks” at the time.
They said: “When we had the Novavax jab they told us it would be a matter of weeks, we are now five months past that.
“Originally it was (the approval) to be in the first part of this year, now it looks like it could be anything until the middle of next year.”
The Echo reported on Friday that hundreds of volunteers struck and wrote a joint letter to Health Secretary Sajid Javid calling for “fair treatment” for the 15,000 participants left in limbo amid approval delays.
EXCLUSIVE: “We have been abandoned”: those who took part in the trial of a vaccine in the North-East struck
In the letter, which was signed by dozens of Hartlepool attendees, they urged him to rectify the situation “immediately” to allow Novavax volunteers to be recognized and to travel freely.
Meanwhile, on Saturday it was reported that England’s Deputy Chief Medical Officer Professor Jonathan Van-Tam had suggested that UK clinical trial data be denied to the EU, which was later denied .
 It will be produced at Billingham, if successful
It will be produced at Billingham, if successful
But a government spokesperson said participating in a Covid vaccine trial should not put them at a disadvantage and the government intended to “take steps” to ensure that remains the case.
The government said the Vaccine Task Force and the Medicines and Health Care Regulatory Agency (MHRA) are now working with Novavax to ensure approval is cleared as “quickly” as possible, as long as possible.
A government spokesperson said: “The COVID-19 vaccine clinical trials have been instrumental in getting us to the point where we have safe and approved vaccines and they are providing critical data to help us respond to this pandemic.
“We are clear that volunteers in officially approved COVID-19 vaccine trials in the UK should not be at a disadvantage with vaccine certification policies, and we commit to taking action on this issue, including by reviewing supplemental immunization guidelines for this group. ”
The government has said that clinical trial participants can now access an NHS COVID Pass for home use.
In addition, they said they should have a letter confirming their participation in the specific vaccine study and confirming that they have the same protected status as someone who has received the approved vaccines.
Novavax had previously admitted to The Echo that the schedules had “changed” and that he hoped to submit his findings for approval in the coming months, although no definitive date has been given.
He said he was now in active talks with various regulatory bodies around the world, including the Medicines and Health Products Regulatory Agency (MHRA), the World Health Organization and the Foods and Drug. United States Administration.
In a statement, he said: “Novavax is aligning with these regulatory authorities on the final requirements to complete these submissions.
“As a result, more work is underway and we now expect these final deposits, including with the MHRA, to occur in close proximity to each other over the next two months.”
He said he was continuing to work “as quickly as possible” to complete submissions to UK regulators.
The Medicines and Health Products Regulatory Agency previously declined to comment citing “commercial sensitivity”.
Are you in the North-East and have been affected by the Novavax trial? If so, send an email to [email protected]
–
Stay up to date with all the latest news on our website, or follow us on Facebook, Twitter and Instagram.
You can also follow our dedicated Teesside Facebook page for all the latest news in the region by clicking here.
For all the major updates from across the region delivered straight to your inbox, sign up for our newsletter here.
Do you have a story for us? Contact our press office on [email protected] or contact 01325 505054
[ad_2]
Source link