[ad_1]
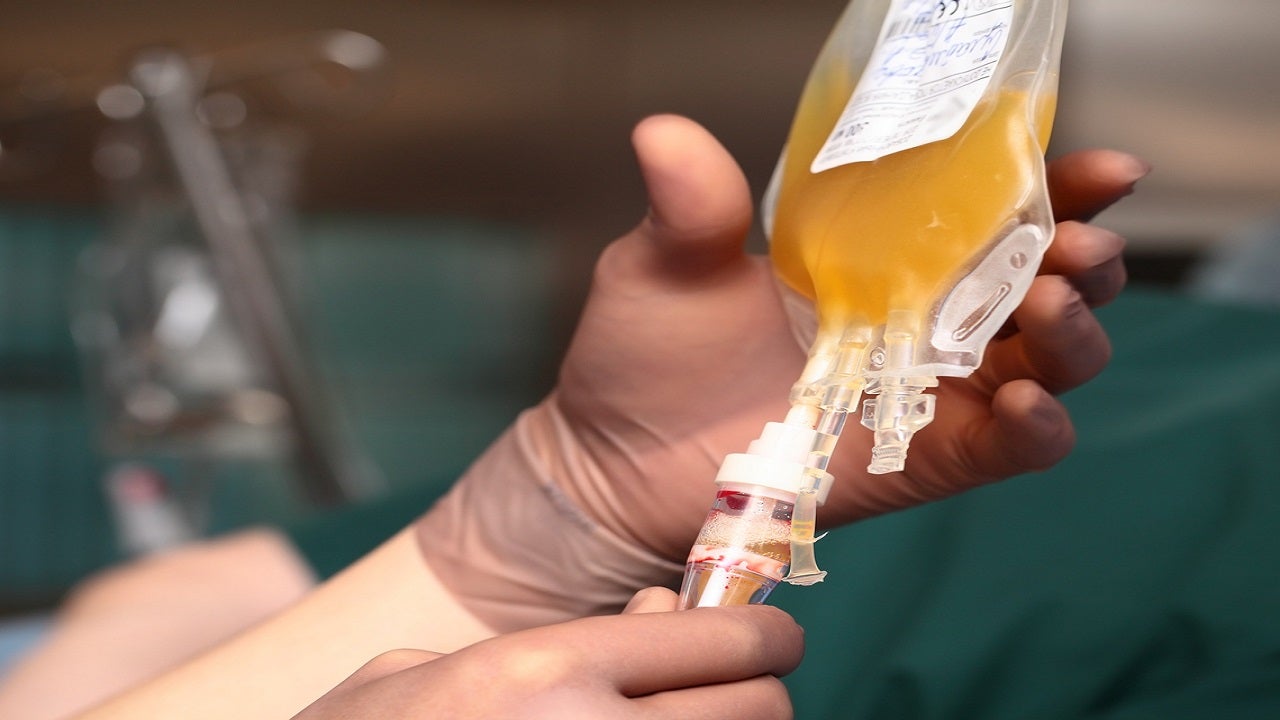
The National Institutes of Health said on Tuesday it had stopped a clinical trial testing convalescent plasma in patients with mild to moderate COVID-19 after independent advice found no difference in hospitalizations, deaths or in preventing progression to serious disease.
An independent data and safety oversight committee met on February 25 to assess the data from the trials and concluded that while the plasma “did not cause any harm, it was unlikely to benefit this group. of patients, “according to a press release. new patients in the study, and it was done “immediately,” the statement said.
CONVALESCENT PLASMA QUESTION AS A TREATMENT FOR CORONAVIRUS IN INDIA: REPORT
“Recent analysis of study data showed no significant difference in the proportion of participants who experienced any of these outcomes. [hospitalization, additional care or death within two weeks]”The statement read.” Even if recruitment continued, it was highly unlikely that this trial would demonstrate that COVID-19 convalescent plasma prevents progression from mild to severe illness in outpatients at the emergency at risk.
The study was launched in August 2020 and aimed to reach 900 patients in 47 hospital emergency departments (ERs) in the United States, but recruited only 511 patients. These patients presented to the emergency room with mild to moderate COVID-19 and had at least one underlying condition that would increase the risk of a severe course of COVID-19 disease, such as heart disease or obesity. The patients exhibited symptoms for several days to a week, but were not sick enough to require hospitalization.
The concept behind the treatment is that the antibodies in the plasma of cured patients could be infused into sick patients in an attempt to improve conditions. Plasma was also used during the 1918 influenza pandemic, the 2003 SARS-CoV-1 epidemic, and the 2009 H1N1 influenza pandemic, according to the NIH.
GET THE FOX NEWS APP
The trial participants were given a unit of plasma or a placebo, and the researchers studied whether the patients needed hospital care, requested additional care, or died within 15 days of starting the trial.
The NIH noted that more than 100,000 people in the United States have already been treated with plasma since the start of the pandemic and that the American Red Cross is actively seeking plasma donations. Some doctors have expressed cautious optimism about treatment last spring, although they are uncertain, especially whether the improvement in patients’ conditions was due to plasma or some other factor.
[ad_2]
Source link