
[ad_1]
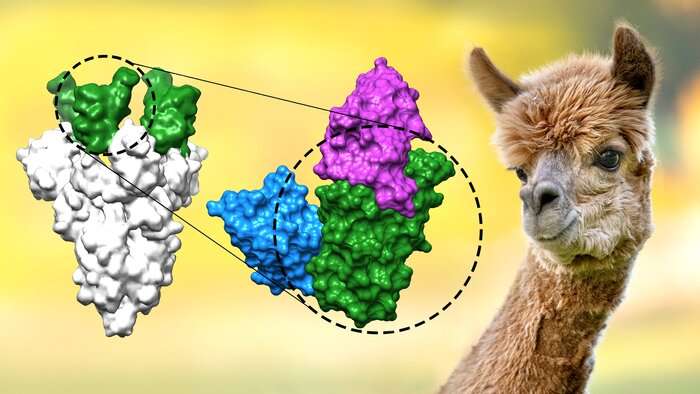
The figure shows how two of the newly developed nanobodies (blue and magenta) bind to the receptor binding domain (green) of the coronavirus spike protein (gray), thereby preventing infection with Sars-CoV-2 and its variants . Nanobodies originate from alpacas and are smaller and simpler than conventional antibodies. Credit: Max Planck Company
Researchers from Göttingen have developed mini-antibodies that effectively block the SARS-CoV-2 coronavirus and its dangerous new variants. These so-called nanobodies bind and neutralize the virus up to 1000 times better than previously developed mini-antibodies. In addition, scientists have optimized their mini-antibodies for stability and resistance to extreme heat. This unique combination makes them promising agents for treating COVID-19. Since nanobodies can be produced inexpensively in large quantities, they could meet the global demand for COVID-19 therapies. The new nanobodies are currently being prepared for clinical trials.
Antibodies help our immune system fend off pathogens. For example, molecules attach themselves to viruses and neutralize them so that they can no longer infect cells. The antibodies can also be produced industrially and administered to critically ill patients. They then act like drugs, relieving symptoms and shortening recovery from the disease. This is an established practice for the treatment of hepatitis B and rabies. The antibodies are also used to treat patients with COVID-19. However, producing these molecules on an industrial scale is too complex and expensive to meet global demand. Nanobodies could solve this problem.
Scientists from the Max Planck Institute (MPI) for Biophysical Chemistry in Göttingen (Germany) and the University Medical Center in Göttingen (UMG) have now developed mini-antibodies (also known as VHH antibodies or nanobodies) that combine all the required properties for a potent drug against COVID-19. “For the first time, they combine extreme stability and exceptional efficacy against the virus and its mutants Alpha, Beta, Gamma and Delta”, underlines Dirk Görlich, director of the MPI for Biophysical Chemistry.
At first glance, the new nanobodies do not differ much from the anti-SARS-CoV-2 nanobodies developed by other laboratories. They are all directed against a crucial part of the coronavirus peaks, the receptor binding domain that the virus deploys to invade host cells. Nanobodies block this binding domain and thus prevent the virus from infecting cells.
“Our nanobodies can withstand temperatures of up to 95 ° C without losing their function or forming aggregates,” explains Matthias Dobbelstein, professor and director of the Institute for Molecular Oncology at UMG. “On the one hand, it tells us that they could remain active in the body long enough to be effective. On the other hand, heat-resistant nanobodies are easier to produce, process and store.”
Single, double and triple nanobodies
The simplest mini-antibodies developed by the Göttingen team already bind up to 1000 times more strongly to the spike protein than previously reported nanobodies. They also bind very well to mutated receptor binding domains of Alpha, Beta, Gamma and Delta strains. “Our unique nanobodies are potentially suitable for inhalation and therefore for the direct neutralization of the virus in the respiratory tract,” said Dobbelstein. “Plus, because they’re very small, they could easily penetrate tissue and prevent the virus from spreading further to the site of infection.”
A “nanobody triad” further improves binding: The researchers grouped three identical nanobodies according to the symmetry of the spike protein, which is made up of three identical building blocks with three binding domains. “With the nanobodies triad, we are literally joining forces: in an ideal scenario, each of the three nanobodies attaches to one of the three binding domains,” reports Thomas Güttler, a scientist in the Görlich team. “This creates a virtually irreversible bond. The triple will not release the spike protein and neutralizes the virus even up to 30,000 times better than single nanobodies.” Another advantage: the larger size of the triad of nanobodies likely delays renal excretion. This keeps them in the body longer and promises a longer lasting therapeutic effect.
As a third design, scientists have produced tandems. These combine two nanobodies that target different parts of the receptor binding domain and together can bind the spike protein. “Such tandems are extremely resistant to viral mutations and the resulting ‘immune escape’ because they bind so strongly to the viral peak,” explains Metin Aksu, researcher in Görlich’s team.
For all variants of nanobodies (monomeric, double or triple), the researchers found that very small amounts are enough to stop the pathogen. If used as a medicine, it would allow a low dosage and therefore less side effects and lower production costs.
Alpacas provide blueprints for mini-antibodies
“Our nanobodies come from alpacas and are smaller and simpler than conventional antibodies,” explains Görlich. To generate the nanobodies against SARS-CoV-2, the researchers immunized three alpacas – Britta, Nora and Xenia from the MPI for Biophysical Chemistry herd – with parts of the coronavirus spike protein. The mares then produced antibodies and the scientists took a small blood sample from the animals. For the alpacas, the mission was then over, as all subsequent steps were carried out using enzymes, bacteria, bacteriophages and yeasts. “The overall burden on our animals is very low, comparable to vaccination and blood tests in humans,” explains Görlich.
Görlich’s team extracted around a billion blueprints of nanobodies from the blood of alpacas. What followed was a perfected laboratory routine over many years: Biochemists used bacteriophages to select the best nanobodies from the large initially large pool of candidates. These were then tested for their effectiveness against SARS-CoV-2 and further improved in successive optimization cycles.
Not all antibodies are “neutralizing”. Researchers in Dobbelstein’s group therefore determined whether and to what extent nanobodies prevent viruses from replicating in cells grown in the laboratory. “By testing a wide range of dilutions of nanobodies, we find out how much is enough to achieve this effect,” explains Antje Dickmanns of the Dobbelstein team. Her colleague Kim Stegmann adds: “Some of the nanobodies were really impressive. Less than a millionth of a gram per liter of medium was sufficient to completely prevent infection. In the case of the nanobody triads, even another twenty-fold dilution was sufficient. “
Also effective against current variants of the coronavirus
During the coronavirus pandemic, new viral variants appeared and quickly became dominant. These variants are often more infectious than the strain that first appeared in Wuhan (China). Their mutated spike protein can also “escape” neutralization by certain initially effective antibodies from infected, cured or vaccinated people. This makes it more difficult, even for an already formed immune system, to eliminate the virus. This problem also affects previously developed therapeutic antibodies and nanobodies.
This is where the new nanobodies show their full potential, as they are also effective against the main coronavirus variants of concern. The researchers had inoculated their alpacas with some of the spike protein from the first known SARS-CoV-2 virus, but remarkably, the animals’ immune systems also produced antibodies that were active against the different variants of the virus. “If our nanobodies prove ineffective against a future variant, we can reimmunize the alpacas. As they have already been vaccinated against the virus, they would very quickly produce antibodies against the new variant, ”says Güttler confidently.
Therapeutic application in sight
The Göttingen team is currently preparing nanobodies for therapeutic use. Dobbelstein emphasizes: “We want to test nanobodies as soon as possible for safe use as a medicine so that they can benefit people with severe COVID-19 and those who have not been vaccinated or cannot develop effective immunity. “The team is supported by technology transfer experts: Dieter Link (Max Planck Innovation), Johannes Bange (Lead Discovery Center, Dortmund, Germany) and Holm Keller (kENUP Foundation).
The SARS-CoV-2 receptor binding domain is known to be a good candidate for a protein vaccine but heretofore difficult to manufacture economically on a large scale and in a form which activates the immune system against the virus. Bacteria programmed accordingly produce poorly folded material. Researchers at Göttingen have discovered a solution to this problem: they have identified special nanobodies that force correct folding in bacterial cells, without obstructing the crucial neutralizing part of the receptor binding domain. This could make it possible to produce vaccines at a lower cost, which can be quickly adapted to new viral variants and can be distributed with simple logistics, even in countries with little infrastructure. “The fact that nanobodies can aid in protein folding was not previously known and is extremely interesting for research and pharmaceutical applications,” says Görlich.
Nanobodies inhibit SARS-CoV-2 infection, including emerging variants
Thomas Güttler et al, Neutralization of SARS-CoV-2 by very powerful, hyperthermostable and mutational tolerant nanobodies, The EMBO Journal (2021). DOI: 10.15252 / embj.2021107985
Provided by the company Max Planck
Quote: Stable and very powerful nanobodies stop SARS-CoV-2 (2021, July 28) retrieved July 29, 2021 from https://medicalxpress.com/news/2021-07-highly-potent-stable-nanobodies-sars- cov-. html
This document is subject to copyright. Other than fair use for private study or research purposes, no part may be reproduced without written permission. The content is provided for information only.
[ad_2]
Source link