[ad_1]
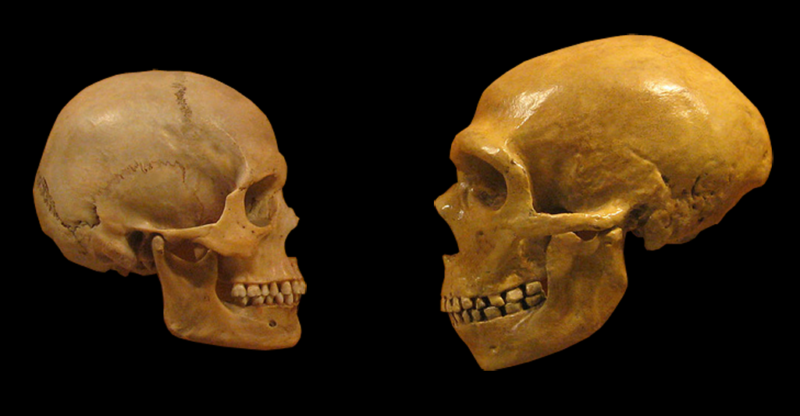
What are the main differences between modern humans and our closest relatives, Neanderthals and Denisovans? For Neanderthals, there doesn’t seem to be an obvious difference. They used sophisticated tools, made art, and established themselves in very harsh environments. But, as far as we can tell, their overall population has never been particularly high. When modern humans arrived at the scene in Eurasia our numbers increased, we spread even more, and Neanderthals and Denisovans ended up being displaced and ultimately extinct.
Thanks to our ability to obtain ancient DNA, we now have insight into the genomes of Neanderthals and Denisovans, which allows us to ask a more specific question: Could some of our differences be due to genetics?
All three species are close relatives, so the number of differences in our proteins is relatively small. But a large international research team has identified one and reinvented it in stem cells obtained from modern humans. And the researchers found that the neural tissue made up of these cells had notable differences from the same tissue grown with the modern human version of this gene.
A not quite great NOVA
As the first step in their work, the researchers had to decide on a gene to target. As we mentioned above, the genomes of the three species are extremely similar. And the similarity only rises when you look at the parts of the genome that code for proteins. An additional complication is that some of the gene versions found in Neanderthals are still found in a fraction of the modern human population. What the researchers wanted to do was find a gene in which Neanderthals and Denisovans had one version and almost all modern humans had another.
Out of tens of thousands of genes, they found only 61 that passed this test. The one they chose to focus on was called NOVA1. Despite the explosive sounding name, NOVA1 was simply named after it was originally found to be associated with cancer: Ventral Neuro-Oncology Antigen 1. A glance into the vertebrate family tree shows that Neanderthals and Denisovans share a version of NOVA1 along with everything from other primates to chickens, which means it was present in the ancestor that mammals shared with dinosaurs.
Yet almost all humans have a different version of the gene (in a search of a quarter of a million genomes in a database, researchers were only able to identify three instances of the Neanderthal version). The difference is subtle – swapping out a closely related amino acid at one place in the gene – but it’s a difference. (For those who care, it’s isoleucine to valine.)
But NOVA1 is the kind of gene where small changes can potentially have a big impact. The RNAs that are used to make proteins are initially made up of a mixture of useful parts separated by unnecessary spacers that must be spliced. For some genes, the different parts can be spliced together in more than one way, allowing distinct forms of a protein to be made from the same starting RNA. NOVA1 regulates the splicing process and can determine which form of several genes is produced in cells where it is active. For NOVA1, the cells in which it is active include many parts of the nervous system.
If that last paragraph was a little confusing, the short version is: NOVA1 can change the types of proteins made in nerve cells. And, since behavior is an area in which modern humans may have been different from Neanderthals, it is an intriguing target for this type of study.
On our nerves
Obviously, there are ethical issues in trying to see what the Neanderthals version would do in actual humans. But certain technologies developed over the past ten years now allow us to approach the question in a very different way. First, the researchers were able to take cells from two different people and convert them into stem cells, capable of developing into any cell in the body. Then they used CRISPR gene editing technology to convert the human version of the gene to a Neanderthal version. (Or, if you’re less charitable, you can call it the chicken version.)
After performing extensive checks which indicated that NOVA1 was the only gene changed by editing, the researchers induced the stem cells to form the typical neurons in the cerebral cortex.
The resulting neural cell clusters were smaller when formed by cells with the Neanderthal version of NOVA1, although these clusters have a more complex surface shape. Cells in the Neanderthal version also grew more slowly and tend to undergo a process that more often ends in cell death. So it was clear that the Neanderthal version altered the behavior of stem cells when they were converted into nerve cells.
Genetic differences were also apparent. The research team looked for all genes that had altered activity (as measured by levels of messenger RNA) in cells with Neanderthals NOVA1. There were a number of them and included key regulators of neural development. And, as expected from a splicing regulator, there were hundreds of genes that saw changes in the way their protein-coding RNAs were being put together.
Many of these genes appear to be involved in the formation and activity of synapses, the individual connections between nerve cells that allow them to communicate with each other. Not surprisingly, this changed the behavior of these connections. Normally, nerve cells in culture form connections and coordinate their activity. In cells with the Neanderthal version of NOVA1, there was less coordination and a higher background of nerve cells emitting random signals.
A matter of context
The results clearly show that the Neanderthal version of NOVA1 is not a good thing for the nerve cells of modern humans. It will still take a bit of work, however, to determine whether all of the changes described here are the product of specific differences between the two forms of the protein or simply a consequence of poor nerve cell health due to poor gene regulation. .
But the researchers also caution against overinterpreting the results in general – although suggestive, these results are not a clear indication that genetic changes make our brains fundamentally different from those of our closest relatives.
The evolution of the human version of this gene has taken place against a background of many other subtle modifications to human genes, either in their coding sequences or (more often) in the sequences that regulate their activity. These changes could potentially offset the adverse effects caused by the differences in activity of the modern human version of NOVA1. Suddenly dropping the original version of the gene again might only produce differences due to the mismatch between the gene and all of these compensations.
So it will take some time to determine what the differences in this gene mean for human and Neanderthal brains. But the main thing is that it is now possible to ask these questions. The technologies used to produce these results did not exist until this century – CRISPR gene editing is less than a decade old. So the mere fact that we know about it is quite astonishing.
Science, 2021. DOI: 10.1126 / science.aax2537 (About DOIs).
[ad_2]
Source link