
[ad_1]
I am often asked how carbon dioxide can have a significant effect on the planet's climate when its concentration is so low (0.041% of the Earth's atmosphere). And human activities are only responsible for 32% of this amount.
I study the importance of atmospheric gases for air pollution and climate change. The key to the strong influence of carbon dioxide on the climate is its ability to absorb the heat emitted by the surface of our planet and prevent it from escaping into space.
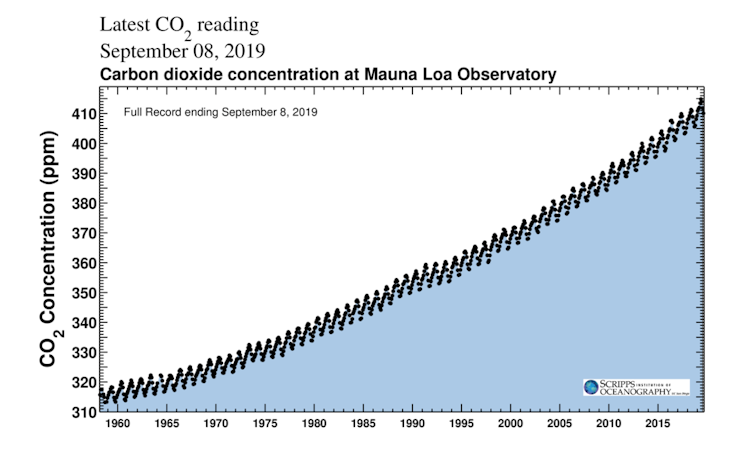
Scripps Institution of Oceanography, CC BY
Early greenhouse science
Scientists who first identified the importance of carbon dioxide for the climate in the 1850s were also surprised by its influence. Working separately, John Tyndall in England and Eunice Foote in the United States discovered that carbon dioxide, water vapor and methane all absorb heat, unlike more abundant gases.
Scientists had previously calculated that the Earth had a temperature of about 33 ° C (59 ° F) higher than normal, given the amount of sunlight reaching its surface. The best explanation for this difference was that the atmosphere held the heat to warm the planet.
Tyndall and Foote have shown that nitrogen and oxygen, which together account for 99% of the atmosphere, have virtually no influence on the Earth's temperature because they do not absorb heat. On the contrary, they found that gases in much lower concentrations were entirely responsible for maintaining the temperatures making the Earth habitable by trapping heat to create a natural greenhouse effect.
A blanket in the atmosphere
The Earth constantly receives energy from the sun and sends it back into space. In order for the planet's temperature to remain constant, the net heat it receives from the sun must be balanced by the outgoing heat it releases.
As the sun is hot, it gives off energy in the form of shortwave radiation mainly in the ultraviolet and visible wavelengths. Because the Earth is much colder, it emits heat in the form of infrared radiation, which has longer wavelengths.
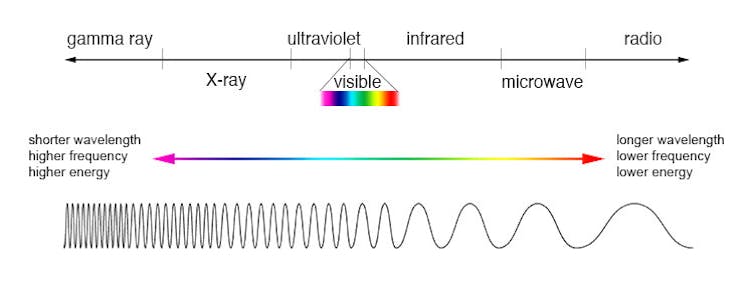
NASA
Carbon dioxide and other heat-trapping gases have molecular structures that allow them to absorb infrared radiation. The bonds between the atoms of a molecule can vibrate in different ways, such as the sound of a piano wire. When the energy of a photon matches the frequency of the molecule, it is absorbed and its energy is transferred to the molecule.
Carbon dioxide and other heat-trapping gases have at least three atoms and frequencies corresponding to the infrared radiation emitted by the Earth. Oxygen and nitrogen, with only two atoms in their molecules, do not absorb infrared radiation.
Most of the sun's rays that arrive from the shortwave flow through the atmosphere without being absorbed. But most of the outgoing infrared rays are absorbed by gases that retain heat in the atmosphere. Then they can release or re-emit this heat. Some return to the surface of the Earth, keeping it warmer than it otherwise would be.
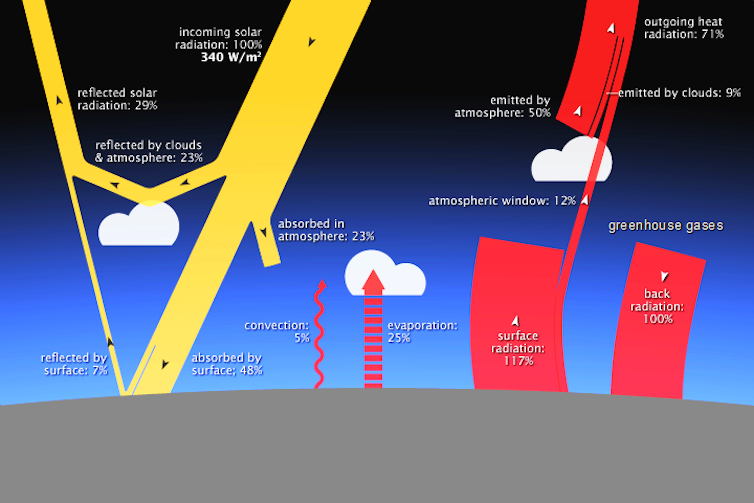
NASA via Wikimedia
Research on heat transfer
During the Cold War, the absorption of infrared radiation by many different gases has been studied extensively. The work was led by the US Air Force, which was developing heat-seeking missiles and had to understand how to detect the heat that was passing through the air.
This research has allowed scientists to understand the climate and atmospheric composition of all planets in the solar system by observing their infrared signatures. For example, Venus is about 470 C (870 F) because its thick atmosphere is composed of 96.5% carbon dioxide.
It has also informed weather forecasts and climate models, allowing them to quantify the amount of infrared radiation retained in the atmosphere and returned to the Earth's surface.
People sometimes ask me why carbon dioxide is important for the climate, because the water vapor absorbs more infrared rays and the two gases absorb at several identical wavelengths. The reason is that the upper earth's atmosphere controls the radiation that escapes into space. The upper atmosphere is much less dense and contains much less water vapor than near the ground, which means that the addition of more carbon dioxide significantly influences the amount of infrared radiation that s & # 39; Escapes into space.
Observe the greenhouse effect
Have you ever noticed that deserts are often colder at night than forests, even if their average temperatures are the same? Without too much water vapor in the atmosphere above the deserts, the radiation they emit easily escapes into space. In wetter areas, surface radiation is trapped by water vapor in the air. Similarly, cloudy nights tend to be warmer than clear nights because there is more water vapor.
The influence of carbon dioxide can be seen in past climate changes. The ice cores of these last million years have shown that carbon dioxide concentrations were high during warm periods – about 0.028%. During glacial periods, when the Earth was about 4 to 7 ° C cooler than in the 20th century, carbon dioxide accounted for only about 0.018% of the atmosphere.
Although water vapor plays a larger role in the natural greenhouse effect, changes in carbon dioxide have resulted in past temperature changes. In contrast, the levels of water vapor in the atmosphere react to the temperature. As the Earth heats up, its atmosphere may contain more water vapor, which amplifies the initial warming in a process called "water vapor feedback". Changes in carbon dioxide have thus had a decisive influence on past climate change.
Small change, big effects
It is not surprising that a small amount of carbon dioxide in the atmosphere can have a considerable effect. We take pills that represent only a tiny fraction of our body mass and we expect them to affect us.
Today, the level of carbon dioxide is higher than ever before. Scientists agree that the average temperature on the surface of the Earth has already increased by about 1 F (2 F) since the 1880s and that increases in carbon dioxide and carbon monoxide. Other heat-trapping gases caused by humans are extremely responsible.
Without action to control emissions, carbon dioxide could reach 0.1% of the atmosphere by 2100, more than triple the level before the industrial revolution. It would be a faster change than the transitions of Earth's past that had enormous consequences. Without action, this small burst of atmosphere will cause big problems.
[[[[Deep knowledge, daily. Sign up for The Conversation newsletter. ]
[ad_2]
Source link