[ad_1]
World food production requires ammonia-based fertilizers. Industrial transformation of atmospheric nitrogen (N2, also known as dinitrogen) in ammonia (NH3) is therefore essential for human life. Despite the simplicity of the molecules involved, the cleavage of the strong nitrogen – nitrogen triple bond (the N≡N bond) in the dinitrogen and the concomitant formation of nitrogen – hydrogen (N – H) bonds pose a difficult challenge to catalytic chemistry. generally involves expensive energy conditions: high reaction temperatures, high pressures, or combinations of reactive reagents that are difficult to handle and that consume too much energy. Write in NatureAshida et al.1 demonstrate that a mixture of samarium mixed with water and associated with a molybdenum catalyst can promote the synthesis of ammonia from dinitrogen under ambient conditions. The work opens avenues for research into the hunt for ammonia processes that operate under ambient conditions and raises the question of what should be the ideal process.
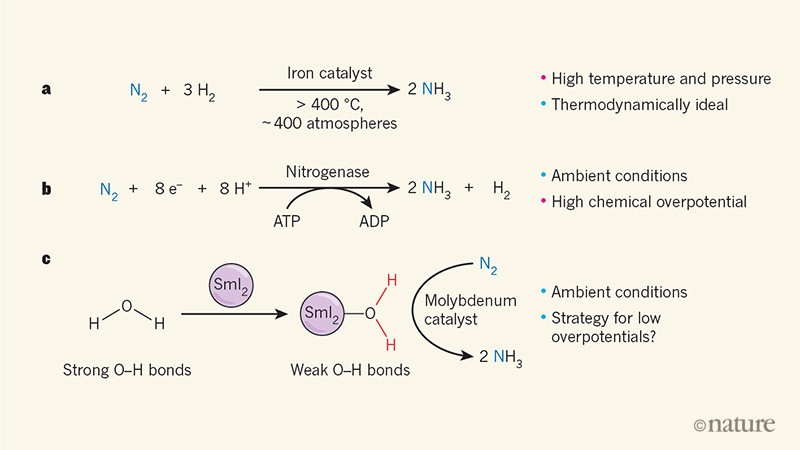
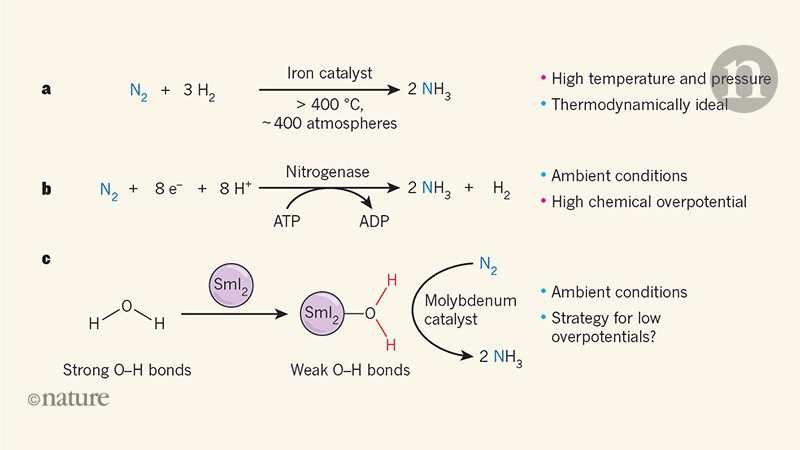
Chemists Fritz Haber and Carl Bosch, chemists Fritz Haber and Carl Bosch, were the first to demonstrate that, motivated by the global shortage of fertilizer in the early twentieth century, then by the shortage of ammunition (l 39; ammonia can be used to make explosives)2 this dinitrogen could be "extracted from the air" and converted to ammonia. In the modern version of the Haber – Bosch process, dinitrogen and hydrogen are combined on a typical iron – based catalyst to produce ammonia (Fig. 1a). Today, global ammonia production is between 250 and 300 tonnes per minute and provides fertilizers that account for nearly 60% of the world's population.3,4.
Modern ammonia synthesis conditions involve temperatures above 400 ° C and pressures of about 400 atmospheres, and are therefore often referred to as "hard". This common misconception has prompted chemists to find "softer" alternatives using new catalysts to lower operating temperatures and pressures. In fact, the search for new catalysts should be inspired by the need to reduce capital expenditures related to the construction of ammonia plants and the need to reduce carbon emissions – not just the synthesis of ammonia. ammonia, but also the production of hydrogen used for manufacturing. the process5.
Chemists have turned to nature for inspiration, as they often do. The family of nitrogenase enzymes is largely responsible for the biological conversion of nitrogen to ammonia (process called nitrogen fixation) and is the source of nitrogen atoms in amino acids and nucleotides, constituent elements of life. Unlike the Haber – Bosch process, nitrogenases do not use hydrogen as a source of hydrogen atoms. Instead, they transfer protons (hydrogen ions;+) and electrons at each nitrogen atom to form N – H bonds (Figure 1b). But although nitrogenases fix nitrogen at room temperature, they use eight equivalents of protons and electrons per molecule of nitrogen (instead of six, the number needed depending on the stoichiometry of the reaction) to provide the thermodynamic force necessary for fixation and other coupled processes.6. This use of excess hydrogen equivalents means that the nitrogenases act with a high chemical potential – they use a lot more energy than is actually needed to provide fixation.7.
The chemists have imitated the nitrogenase reaction by adding proton and electron sources to metal-containing complexes that contain bound dinitrogen. For example, workers in the same group as Ashida et al. Posted previously8 Molybdenum complexes that catalyze fixation in this manner, producing up to 230 molecules of ammonia per molybdenum complex. However, the associated overpotentials are substantial (reaching nearly 300 kilocalories per mole of dinitrogen, in some cases).9. Seen in this light, the Haber – Bosch process is about to become a thermodynamically ideal process for the synthesis of ammonia, and it is not as energetically hard as it is sometimes claimed.
A challenge for catalysis researchers is to combine the best of biological and industrial approaches to nitrogen fixation, that is, to find a process that works close to ambient temperature and pressure, which has a minimal chemical overpotential and does not require a capital-intensive plant. to make ammonia on a large scale. This is a great challenge because no combination of acids (proton sources) and reducing agents (electron sources) has been found to provide a thermodynamic force of fixation equivalent to that of hydrogen, and sufficiently reactive to form N – H bonds from nitrogen at ambient temperature or at a temperature close thereto.
But what if, instead of working separately, sources of protons and electrons could be brought together? Ashida et al. have adopted this strategy and thus signal what could be a fundamentally new approach to catalytic synthesis of ammonia. They use a phenomenon called weakening of the linkage induced by coordinationten, which results from the interaction of samarium diiodide (SmI2) and water (Fig. 1c).
Water that is not part of a chemical complex contains strong oxygen – hydrogen (O – H) bonds that are difficult to split. But when the oxygen atom in the water coordinates (gives its unique pair of electrons) to SmI2the O – H bonds are weakened and the resulting mixture becomes a powerful source of hydrogen atoms, an excellent source of protons and electrons. Ashida et al. use this source of hydrogen atoms with a molybdenum catalyst to fix the nitrogen. A considerable weakening of the links induced by the coordination has already been measured in SmI2Mixtures-water, and used to create carbon-hydrogen bonds11,12.
The extension of this idea to catalytic synthesis of ammonia is remarkable for two main reasons. First of all, it is remarkable that the molybdenum catalyst facilitates the synthesis of ammonia in aqueous solution, because the molybdenum complexes are often degraded in water. Secondly, the coordination-induced weakening of the linkages provides a new way to fix nitrogen under ambient conditions, thus avoiding the use of potentially dangerous combinations of proton and electron sources – such combinations can occur. ignite spontaneously. The authors' approach also works when ethylene glycol (HOCH)2CH2OH) is used in place of water, which broadens the range of hydrogen atom sources for ammonia production.
Ashida et al. Propose a catalytic cycle in which the molybdenum catalyst first coordinates with the dinitrogen and cleaves the N≡N bond to form a nitrido molybdenum complex (which contains a molybdenum-nitrogen triple bond). The smi2The water mixture then delivers equivalents of hydrogen atoms to this complex, finally producing ammonia. The formation of N – H bonds with nitrido molybdenum complexes poses a considerable thermodynamic challenge, since N – H bonds are also weakened when bound to molybdenum, as noted by our group.ten; this effect is a source of chemical overpotential. The smi2 not only facilitates the transfer of hydrogen atoms, but also keeps the metal in a reduced form and prevents the harmful formation of molybdenum oxide in aqueous solution.
The reported method presents considerable operational challenges that currently make ammonia synthesis impracticable: SmI2 is used in large quantities, which generates a lot of waste; the separation of ammonia from aqueous solutions is energetically expensive; and a chemical overpotential of about 140 kcal mol-1 remains. Nevertheless, the work of Ashida and his colleagues creates a playground in which chemists can explore methods of ammonia synthesis. Future research should focus on finding alternatives to the SmI2, based on metals more abundant than samarium, promotes the weakening of the coordination-induced bonds, allows the formation of N-H bonds and reduces the energy costs related to the manufacture of ammonia from ## EQU1 ## 39, air and water.
Sign up for the everyday Nature Briefing email
Stay abreast of what matters in science and why hand-picked Nature and other publications around the world.
S & # 39; register
[ad_2]
Source link