[ad_1]
The field of research on Alzheimer's disease is full of disappointment. Just last week, another drug failed Phase III clinical trials, continuing the 15-year loss since the introduction of a new Alzheimer's disease treatment approved by the Food and Drug Administration. Drug Administration.
But a team of scientists this week said they discovered the first evidence in the mouse that a class of drugs could attack Alzheimer's disease and similar disorders from a different angle than previous attempts.
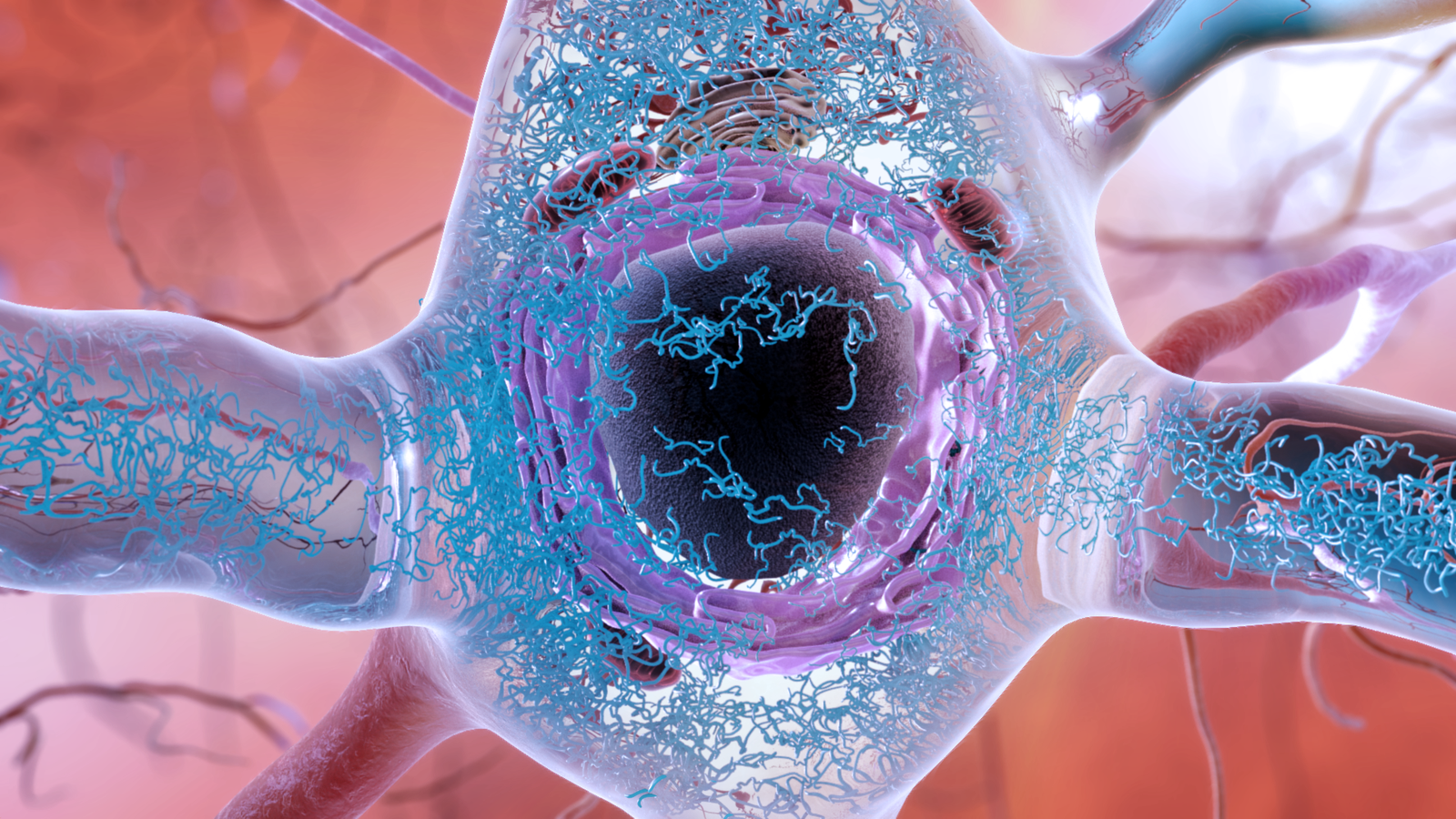
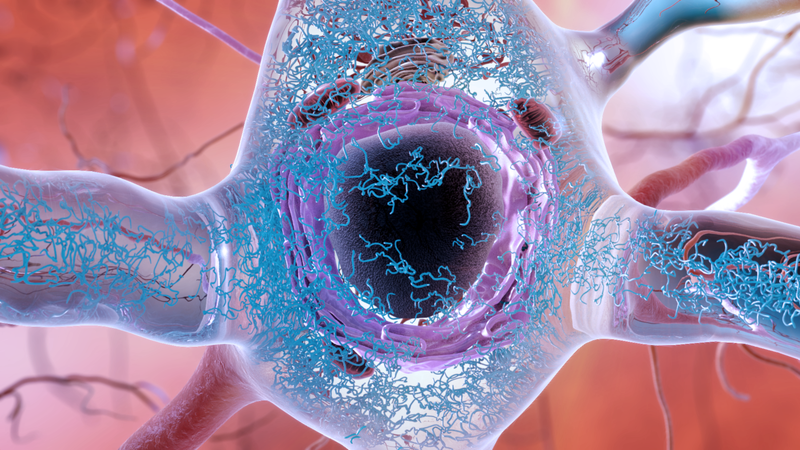
The question of the cause of Alzheimer's disease is a question without simple answers. We know that two proteins naturally produced in the brain, beta-amyloid and tau, are inextricably linked to the neurological disorder. In a person with Alzheimer's disease in its own right, abnormal clusters of both proteins, called plaques and tangles, form and enclose the brain (the plaques are found mainly in the space between nerve cells, while tangles are formed mainly in nerve cells). But we do not know if one, both or none of these structures is primarily responsible for the progressive or even fatal brain damage seen with Alzheimer's disease.
The theory of the most widely recognized disorder is that beta-amyloid is the main villain, in part because plaques appear to appear before tau tangles. Scientists and pharmaceutical companies have therefore put their hopes on drugs capable of breaking down these plaques or preventing their accumulation in the brain. But after testing these anti-amyloid drugs failed to significantly improve the symptoms or slow the progression of the disease. Often the failure of the drug becomes evident only after the human test.
This drug targets a different target from most of these high profile trials. But maybe even tau protein might not prove to be the right target.
The most recent unsuccessful trials of aducanumab, which have been Biogen and Japanese pharmaceuticals Eisai last week were particularly discouraging. Aducanumab appeared to be better at healing than the previous medications and was tested in patients with the earliest clinical stages of Alzheimer's disease – two factors that should have increased its chances of success. These failures have once again prompted the scientific community to ask for reconsideration and even change of priorities and resources, far from the amyloid hypothesis of Alzheimer's disease, as it is called.
The researchers behind this study, published Wednesday in Science Translational Medicine, have been pursuing one of these alternative leads for years.
Their research, led by Kenneth Kosik, a neurologist at the University of California at Santa Barbara, focused on tau, the main driver of Alzheimer's disease rather than amyloid. Although tau tangles later appear in the disorder development, some research has shown that the abnormal tau spread is better correlated with the visible progression of the disease than amyloid (however, plaques may end up in the brains of people without visible dementia). ). And we already know that there are other neurological disorders, such as frontotemporal dementia, which are almost certainly caused by entanglements of tau. Many people with frontotemporal dementia have also inherited genetic mutations involving the production of tau, which greatly increases the risk of entanglement.
"In Alzheimer's you can debate the problem, but with these cases, there is no doubt," Kosik told Gizmodo.
It is by studying these mutations of tau that Kosik and his team made a discovery. They claim to have found in cells a regulatory path never before seen that could trigger the degradation of a healthy tau. This pathway appears to work via a protein called Rhes, which belongs to a larger family of proteins called Ras. The Ras family is itself regulated by an enzyme called farnesyl transferase. And coincidentally, there are already known drugs to inhibit farnesyl transferase.
These farnesyl transferase inhibitors (FTIs) were originally developed as anti-cancer drugs, since mutated Ras protein is also commonly found in tumors and some have even been tested. on the man. Although they have been deemed safe enough to be used, they have not been approved for cancer treatment. Kosik and his team, however, hypothesized that FIT could be re-used to treat tau-related brain disorders, including Alzheimer's disease.
Until now, their early work on mice and human neurons (developed from the stem cells of people with genetically linked frontotemporal dementia) seems to confirm their well-educated intuition. When they administered an FTI, called lonafarnib, to mice raised with a form of frontotemporal dementia, the progression of the symptoms of dementia significantly slowed down. The brains of the treated mice also exhibited less abnormal tau and inflammation than the control mice. And in a petri dish, damaged human neurons were better able to produce healthy tau once exposed to lonafarnib.
Of course, this would hardly be the first time that animal studies have provided a glimmer of hope for a potential new treatment for dementia. But Kosik noted that one of the benefits of Ionafarnib, compared to other experimental drugs, is that it has already been well studied.
"It's a medicine that's safe for humans. This means that the product can be reused for people with entanglement-related diseases, "Kosik said. And I think this has to be one of the next steps. "
Despite this optimism, the Kosik team had to face obstacles in the study of the drug. Lonafarnib is also being investigated for use in the treatment of rare disease in fast aging states, with promising clinical trials that will likely pave the way for FDA approval. But Kosik says the drug's makers, Eiger BioPharmaceuticals, have refused to provide more of the drug to his team for further research on humans. If lonafarnib can no longer be used, he added, his team should find other candidates for FTI.
Logistics aside, Kosik is well aware of the recent dismal progress in finding a new treatment for neurological disorders such as Alzheimer's disease. But he hopes that he and the others have and will continue to learn from the mistakes made on the ground by pursuing their own research.
"This drug targets a different target from most of these high-profile trials. But maybe even tau protein might not prove to be the right target. Maybe we should look at other aspects of the disease, such as inflammation, "he said.
Despite his own theory, Kosik does not approve of completely getting rid of amyloid, but rather being smarter about how we continue research on Alzheimer's disease in general and the multiple Potential treatment pathways that are strongly supported by basic research.
"The lesson we should draw from these trials is not to close a whole aspect of the disease study, but to re-evaluate our approach to these clinical trials in the first place," Kosik said. "I am also a clinician and I understand that when a patient begs for something, anything that might help, there is a great temptation to try something. But many of these tests are Hail Mary. What we really need, more than anything now, is a deeper understanding of the cellular biology underlying this disease. "
[ad_2]
Source link