[ad_1]
Coronavirus vaccine distribution could begin “shortly after” a Food and Drug Administration panel meeting on December 10 to review Pfizer’s application for emergency use authorization (EUA) Health and Human Services Secretary Alex Azar said Tuesday.
Speaking to reporters for an update on Operation Warp Speed, the federal government’s vaccine task force, Azar said Pfizer’s vaccine would be evaluated by the Vaccines and Related Biologics Advisory Committee. from the FDA at the December 10 meeting.
Azar also said Deputy Surgeon General Rear Admiral Erica Schwartz had contacted President-elect Joe Biden’s transition team and briefed them on the task force’s plans, given the General Service Administration decision Monday to initiate the official transition process.
The FDA vaccine advisory committee provides advice on whether or not to authorize the vaccine for emergency use, but the final decision is made by the commissioner. The agency said in a statement last week announcing the meeting that it will be able to “thoroughly assess the data and information submitted in EUA’s application” by December 10, and that it will be prepared for a “solid” discussion at the public meeting.
“The FDA has been preparing for several months for the USA review for COVID-19 vaccines and stands ready to do so as soon as an EUA application is submitted,” Commissioner Stephen M. Hahn said in the statement . “While we cannot predict how long the FDA review will take, the FDA will review the application as quickly as possible, while always doing it in a thorough and science-based manner, so we can help make available a vaccine that the American people deserve as soon as possible. “
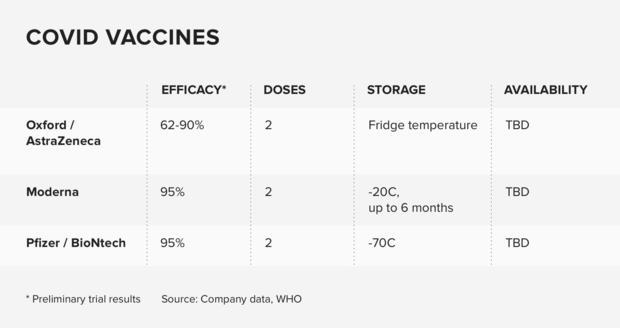
Pfizer and its German partner BioNTech announced that its vaccine appears to be 95% effective in preventing mild to severe COVID-19 on November 18. A few days later, the company formally submitted an application for EUA to the FDA. The move sparked a process that could potentially mark the beginning of the end of the pandemic, after a long and harsh winter.
“We have an end in sight, and we have a much happier vacation in our future,” Azar said Tuesday.
General Gustave Perna, operational manager of Warp Speed, said initial shipments would total 6.4 million doses, with a target of 40 million doses distributed by the end of the year.
Distributing any possible vaccine will prove to be a complex logistical challenge unlike any in recent memory. Azar said Operation Warp Speed began making test shipments to its network this week to ensure a “seamless logistics operation” once a vaccine is approved.
The task force has not officially announced who will be the first to receive a vaccine, but Azar has said that “grassroots groups” such as healthcare workers and residents of nursing homes will take priority. He said the administration expects the general public to be able to get vaccinated “by the second quarter of next year,” but added that supply chains are “growing steadily.”
He described the federal government’s role as the “air traffic controller” of vaccine allocation, and said governors will ultimately determine how the vaccine is distributed in each state. While individual governors feel the need for a more personalized distribution – based on community hot spots within a state – “they are completely in the driver’s seat,” Azar said.
The health secretary noted, however, that the current spread and vulnerability to the disease across the country made per capita distribution the most attractive approach for the task force. “We thought it was better to keep it simple,” he said.
The coronavirus is spreads from coast to coast and killing over 1,500 Americans every day. The morgue overflow in El Paso, Texas necessitated the the national guard must be called in to help. In Ohio, more than 4,300 people were in hospital with symptoms related to COVID-19 on Monday, an increase of 59% from just two weeks ago. Hospitalizations have also increased in New York, up 128% in three weeks – from 1253 earlier this month to 2856.
Due to the low temperatures needed to store the vaccines, Azar said the current plan is to distribute initial orders direct from the manufacturer with 975 doses in each, followed by a second dose 21 to 28 days later. Pfizer’s vaccine should be stored at -80 degrees Celsius for 20 days, while Moderna’s vaccine can be stored at -20 ° C for up to 30 days.
The secretary said vaccine developments offer hope, but the news doesn’t mean Americans should give up their guard – especially during the holiday season.
“We want to make sure everyone is there for Thanksgiving next year,” he said.
[ad_2]
Source link