
[ad_1]
Ochsner Health is recruiting Louisiana children aged 12 to 17 in US trial of Pfizer coronavirus vaccine, as drugmakers near regulatory approvals for their vaccines use by adults begin the process of test them in children.
Currently, the first two vaccines expected to receive clearance from US regulators, Pfizer and Moderna, may get the green light this month. But the authorization is only set to make the drug available for use in people over 18 years old.
In order to receive authorization for use in children – a crucial step in achieving collective immunity in the population – Moderna and Pfizer are now seeking younger participants in the trial. Ochsner plans to recruit 100 children for the Pfizer trials in Louisiana, serving as one of 24 sites across the United States that in total will aim to recruit 2,600 patients.
The Moderna vaccine trial plans to recruit 3,000 children, although it has not announced when it might start recruiting or proposed sites.
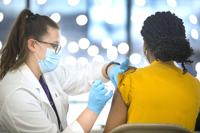
To decide: who is a frontline health worker?
Dr William Lennarz, chairman of Ochsner’s Pediatric Systems and lead physician for the study, said on Friday that so few children have developed severe symptoms of the coronavirus, they can spread the disease to older family and friends and more susceptible to disease. That is why their vaccination is essential to end the pandemic.
“Children themselves are at very low risk, but anyone they come in contact with is definitely susceptible to contracting the infection from children as they are from adults,” Lennarz said.
Children who enroll in the Pfizer trial at the Ochsner campus in Jefferson Highway will receive two doses of the vaccine three weeks apart. Ochsner started recruiting participants in mid-November and has already identified enough patients in the 16-17 age group.
Parental consent is required for study participants, which is expected to last two years. But if all goes according to plan, there should be enough data to show safety and effectiveness in children over 12 years of age by June 2021, according to Dr. John Schieffelin, an expert in pediatric infectious diseases. at Tulane University which is not involved in the study.
About 12% of coronavirus cases are identified in children, according to the American Academy of Pediatrics. Although symptoms are usually mild, at least 138 have died.
Children will constitute a necessary population to be vaccinated to achieve collective immunity, at which point the virus will no longer be able to easily circulate in the community. Scientists estimate that around 60% of a community will need protection to prevent the virus from spreading, and some adults and children may not be able to get vaccinated due to other health concerns – or refuse to be vaccinated.
In Louisiana, children make up nearly a quarter of the state’s 4.6 million people.
“Children, especially adolescents, are at high risk of having an asymptomatic infection, so in the long term, to control this virus we absolutely need to vaccinate children,” said Schieffelin.
Before Admiral Brett Giroir spoke at a press conference in Baton Rouge on Wednesday, he had an important stop to make: the local, independentl…
The vaccine trial at Ochsner involves Pfizer’s mRNA vaccine, which does not contain a weakened or dead version of the virus like many other vaccines.
Instead, the mRNA contains a code that tells the body to make a spike protein exactly like the one the real coronavirus uses to get into cells. When the body recognizes the harmless spike protein, it begins to make antibodies against it. When the real coronavirus enters the body, the body’s immune system has already learned to fight it.
Pfizer said its data indicates that the vaccine is 95% effective in adults, without serious side effects. Among adolescents, Schieffelin does not expect big surprises.
“There is no reason to believe that children in this age group that they are testing will react any differently from young adults in their twenties and even thirties,” he said.
Still, that could change once the trials are extended to younger children, he added.
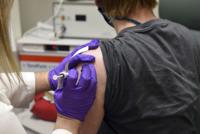
Drugmaker Pfizer Inc.’s announcement on Monday that its coronavirus vaccine may soon be ready for federal approval placed a …
Children under the age of five have an immune system that is still developing. Because of the amount of exposure to new viruses and the number of recommended vaccines they receive, they tend to have “strengthened immune systems,” Lennarz said.
Some have speculated that this is why children don’t seem to be very sick with COVID-19 – and that when they do, the immune system can overdrive and trigger a serious reaction known as syndrome. multisystem inflammatory disease in children, or MIS-C.
The vaccine will likely not be tested in young children until next year, when there is more data in older children. In older adults, it appears to be both effective and safe.
But the trial, like any other study, is not without risk. Children can have different side effects than adults. They might also experience side effects to a greater degree.

Vaccines for the coronavirus are in the process of being approved in the United States, and officials across the country and Louisiana are preparing for the massive task of …
“This virus has humiliated us all time and time again and has proven us wrong time and time again,” Schieffelin said. “That’s why we have to do some testing.”
Those interested in signing up for the trial can email [email protected] for more information.
Emily Woodruff covers public health for The Times-Picayune | New Orleans lawyer as a member of the Report For America corps.
[ad_2]
Source link