[ad_1]
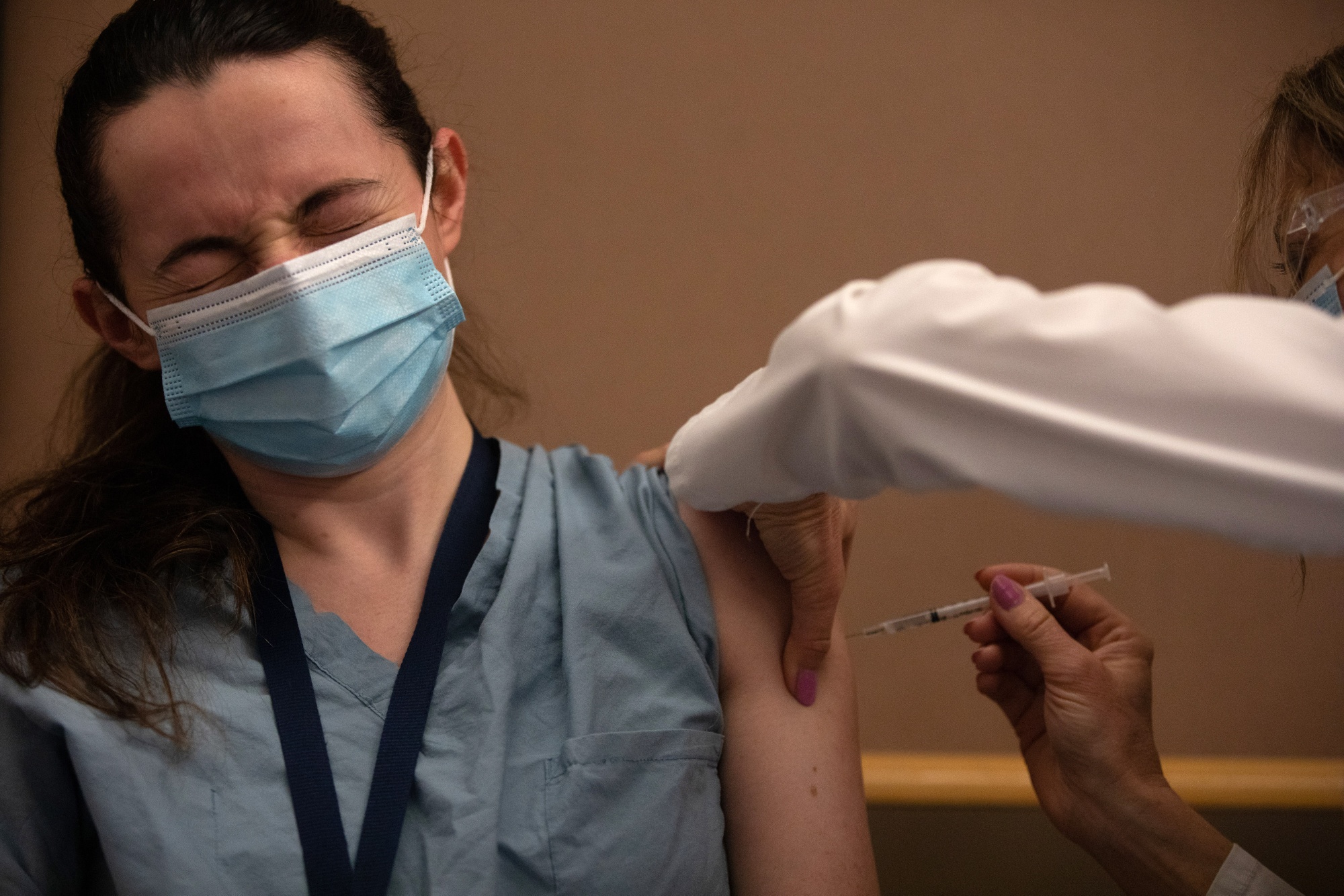
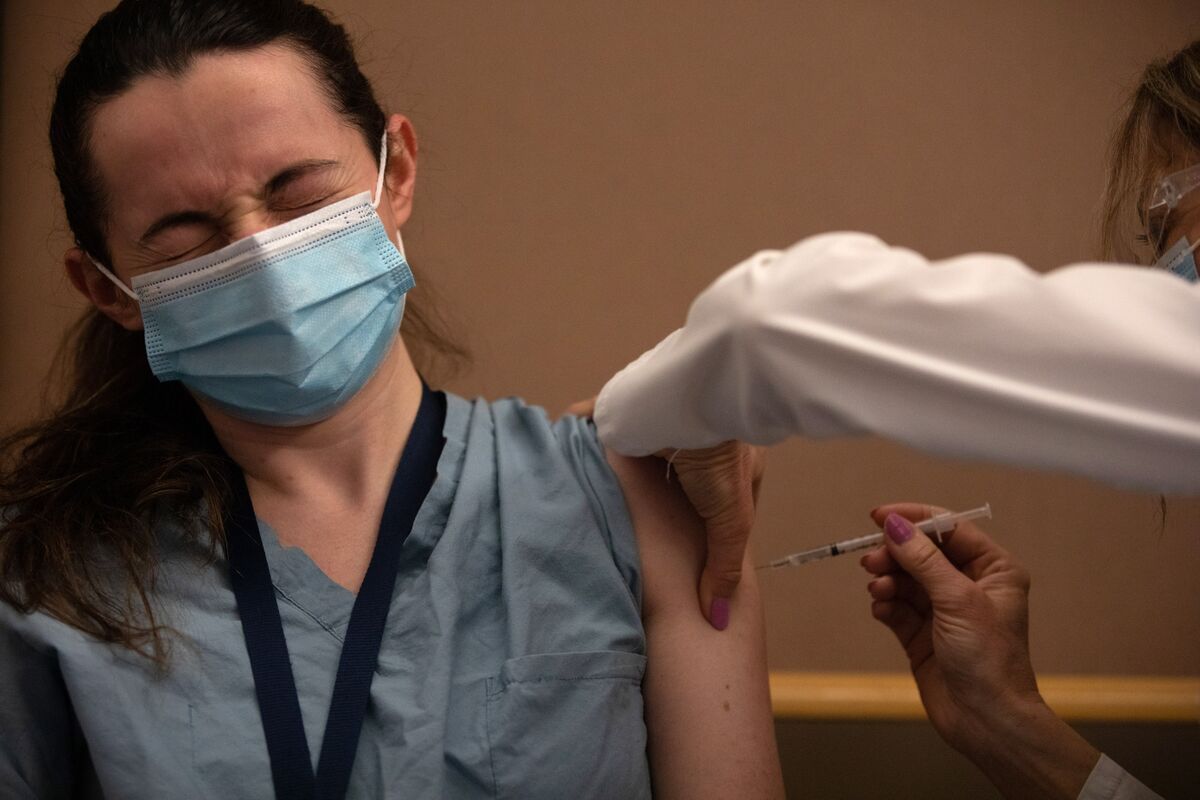
A healthcare worker receives the Pfizer-BioNTech Covid-19 vaccine at the Michigan Medicine Facility in Ann Arbor, Mich., Dec. 16.
Photographer: Emily Welcoming / Bloomberg
Photographer: Emily Welcoming / Bloomberg
Some nurses and emergency responders have expressed reluctance to take the new coronavirus vaccine, a reflection of the unease that U.S. officials hope to overcome by stepping up the nationwide vaccination effort.
For months, polls have shown widespread skepticism about the vaccine after the Trump administration’s efforts to release it before the November election. Public health officials say people’s concerns have subsided since then, but the launch of vaccinations this week made it clear that some health workers and first responders remain reluctant to get vaccinated.
While there are still detractors of the vaccines, the reluctance of members of the medical community could deter the general public from obtaining it despite the safety record and the over 90% effectiveness rate of the initial vaccines. Of the more than 20,000 trial participants who received Pfizer Inc., serious side effects were rare.
In many cases, health workers applauded the arrival of the first doses to fight a virus that has killed more than 308,000 Americans. And the first shots are fired as the United States continues to break death records – more than 3,000 a day.
Still, some young medical workers are reluctant because of widespread but unfounded rumors of possible side effects such as infertility. The American College of Obstetricians and Gynecologists recommend that Covid-19 vaccines not be refused to pregnant women. The group suggests that doctors talk to women about the risk of Covid-19 and the safety of the vaccine for the pregnant patient and the fetus. Pregnant patients are at a higher risk of developing serious illness from Covid-19, according to the United States Centers for Disease Control and Prevention.
Others are worried about the historic speed of the vaccine’s arrival. Pfizer’s vaccine – and one made by Moderna Inc., which is expected to get US clearance as early as Friday, was developed a few months after the pandemic began earlier this year. Government’s Operation Warp Speed program has helped speed up delivery of Covid-19 vaccines and treatment
Vaccine development usually takes much longer – the license can often take 10 years or more, according to the CDC. The Food and Drug Administration has rigorous standards for the approval of vaccines because they are widely administered to healthy people.
Allergic reactions
Concerns were fueled this week by reports that two people in Alaska who received the vaccine suffered rare allergic reactions, which have also been seen in recipients in the UK. Pfizer and US drug regulators are now the review of information for the use and monitoring of the company’s Covid-19 vaccine developed with BioNTech SE.
A national A survey by the American Nurses Foundation in October found that nurses were almost evenly divided when asked if they would be voluntarily vaccinated against the virus, with 36% saying no, 34% saying yes and 31% unsure. A surge of medical groups – including a Twitter hashtag, #IGotTheShot, where health workers post their photos, seems to improve those numbers.
Riverside Health System in Virginia regularly polled its workers to see if they would take it. Acceptance has grown as more data has become available, said Cindy Williams, director of pharmacy at Riverside. Since the last poll about two weeks ago, some people who indicated they would not be taking a vaccine have since emailed them asking if they could change their answer to yes, Williams said in an interview on Wednesday. .
‘Too fast’
But there is still work to be done. At the Loretto Hospital in Chicago, where the city’s first Covid-19 vaccine was administered on Tuesday, 40% of staff are not planning to get it, according to a survey this month.
Sherrie Burch, 56, a room clerk at Loretto, is baffled by how quickly the Covid-19 vaccine has been developed, given the time medical developments typically take. And that makes her nervous. “It happened too fast for me,” Burch said, adding that her children, grandchildren and 76-year-old mother were also not planning to get it. “It’s the fear of the unknown.”
Burch wants more details on vaccine research and long-term side effects. She plans to wait at least a few months to see how her colleagues react to the shooting. Until then, she will continue to mask herself, distance herself, and wash her hands.
Some nurses, respiratory therapists and technicians at Loretto are also stepping down, said Nikhila Juvvadi, clinical director of the hospital who was the first person to administer the vaccine in Chicago. At a public staff meeting on Wednesday, she explained the science of how the Covid-19 mRNA vaccine works.
Such vaccines do not contain the live virus that causes Covid-19 and therefore do not pose a risk of exposing someone to the virus, according to the CDC. Instead, they contain genetic instructions that teach cells how to make antigens that trigger an immune response.
Increase acceptance
Juvvadi hopes the acceptance rate will increase at the hospital with more awareness. “We want to listen,” said Juvvadi, who is among the leaders of the safety net hospital, trying to answer questions and encouraging doctors to show they are getting the vaccine.
She did a conversion this week: Latanya Holmes, who cooks and delivers food to hospital patients. Holmes, 36, was nervous about the potential side effects and arrested Juvvadi in the cafeteria to discuss her concerns. Now she is considering getting the vaccine, but understands the reluctance. “I think it’s the lack of knowledge,” Holmes said.
Workers from other parts of the country are also affected.
In Maine, about 40% of the staff and 30% of residents of the state’s largest nursing homes seem unwilling to be vaccinated, the Maine Health Care Association found after an “informal discussion” with operators.
“Without official polls, it’s difficult to know how accurate this picture is, and we expect these percentages to increase with better education and awareness,” said Nadine Grosso, the organization’s director of communications. “At the end of the day, we know immunization is essential to safely reopening our long-term care facilities.”
Fear of firefighters
A significant number of New York firefighters are also reluctant. Anthony Almojera, paramedical lieutenant who is vice president of Local 3621 of FDNY’s EMS agents union said about 30% of its members resist getting the shot, judging from group chats and social media monitoring.
Five of its union members have died from Covid-19 this year, Almojera said, and about three dozen have been long-term disabled, including two at home on oxygen. Almojera said he was going to take it “to protect me and the people around me, and I love to travel. And I know that proof of vaccination will be a standard for international travel in the future.
He said the biggest concern was the speed of vaccine approval: “Who wants to be a guinea pig?” Almojera is trying to allay union members’ concerns by noting that the Covid vaccine is unique. “He’s been working around the clock for six or seven months with almost unlimited resources,” Almojera said.
Yet some remain impassive. Jonathan Damato, 41, a paramedic in New York for 21 years, is not an anti-vaxxer. He gets the flu shot every year and trusts the life-saving potential of the measles, mumps and polio vaccines. Its station carries around 50 or 60 Covid ambulances per week – people with high fever and shortness of breath.
“I know the virus is real,” said Damato, who has a 4-year-old son with health problems. But “until I see that it’s really safe for me or my kids, I’m not going to take it.”
For others, braving a blow in the arm might help make work less stressful. At the Billings, MT fire department, there is “a bit of apprehension, but no one I’ve heard of is turning it down,” said Cameron Abell of Local 521 of the International Association of Fire Fighters. “It’s hard to wear a mask and take a step back in a job where we really rely on each other socially.”
– With the help of Vincent Del Giudice and John Tozzi
[ad_2]
Source link