
[ad_1]
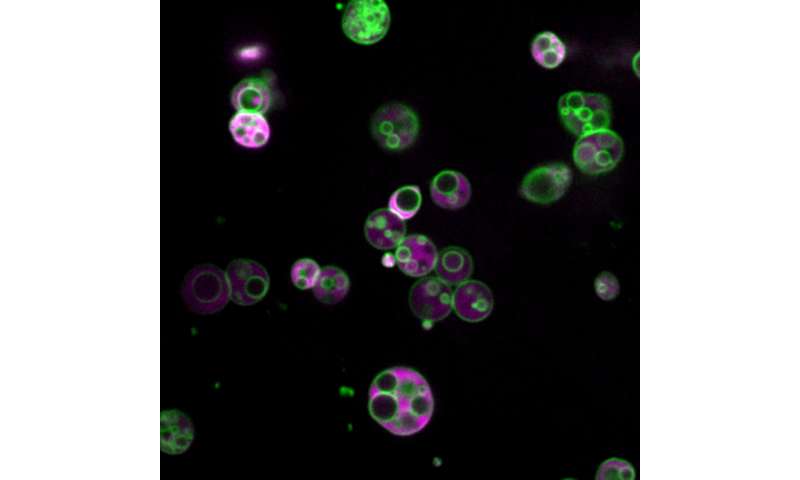
Membrane-separated compartments are visible within the peroxisomes of 4 day old Arabidopsis thaliana plant cells in this image from a confocal microscope. The cells have been genetically engineered to produce fluorescent proteins both in the membranes (green) and in the lumen (magenta) of peroxisomes. (Image courtesy of Zachary Wright / Rice University) Credit: Zachary Wright / Rice University
During his first year of graduate school, Rice University biochemist Zachary Wright discovered something hidden inside a common piece of cellular machinery that is essential to all higher order life. , from yeast to humans.
What Wright saw in 2015 – subcompartments within organelles called peroxisomes – is described in a study published today in Communications of nature.
“This is, without a doubt, the most unexpected thing our lab has ever discovered,” said study co-author Bonnie Bartel, Ph.D. of Wright. advisor and member of the National Academy of Sciences. “It forces us to rethink everything we thought we knew about peroxisomes.”
Peroxisomes are compartments where cells convert fat molecules into energy and useful materials, such as myelin sheaths that protect nerve cells. In humans, peroxisome dysfunction has been associated with serious metabolic disorders, and peroxisomes may have broader significance for neurodegeneration, obesity, cancer, and age-related disorders.
Much is still unknown about peroxisomes, but their basic structure – a granular matrix surrounded by a bag-shaped membrane – was not in question in 2015. Bartel said that was one of the reasons for which Wright’s discovery was surprising.
“We’re geneticists, so we’re used to unexpected things. But generally they don’t come in Technicolor, ”she said, referring to another surprising thing about Wright’s discovery: beautiful color images that show both walls of the peroxisome subcompartments and their interiors. The images were made possible by bright fluorescent reporters, glossy protein tags that Wright used for the experiments. Biochemists modify genes in model organisms – Bartel’s lab uses Arabidopsis plants – to label them with fluorescent proteins in a controlled fashion that can reveal clues about the function and dysfunction of specific genes, including some that cause disease in humans, animals and plants.
Wright, now a postdoctoral research associate in Bartel’s lab, was testing a new reporter in 2015 when he spotted the peroxisome sub-departments.
“I never thought Zach had done anything wrong, but I didn’t think it was real,” Bartel said. She believed the images must have been the result of some sort of artifact, a feature that didn’t really exist inside the cell, but had been created by experience.
“If this was really happening, someone would have noticed by now,” she recalls thinking.
“Basically from that point on, I was trying to figure them out,” Wright said. He checked his instruments, replicated his experiments, and found no evidence of an artifact. He gathered more evidence of the mysterious sub-compartments and ended up in Fondren’s library, browsing through ancient studies.
“I revisited the very old literature on peroxisomes from the 1960s, and saw that they had observed similar things and just didn’t understand them,” he said. “And that idea was just lost.”
There were a number of references to these internal compartments in studies from the 1960s and early 1970s. In each case, investigators focused on something else and mentioned the sighting in passing. And all of the observations were made with transmission electron microscopes, which fell out of favor when confocal microscopy became widely available in the 1980s.
“It’s just a lot easier than electron microscopy,” Bartel said. “The whole field started doing confocal microscopy. And in the early days of confocal microscopy, proteins just weren’t that bright.”
Wright was also using confocal microscopy in 2015, but with brighter reporters that made it easier to resolve small features. Another key: he was examining the peroxisomes of Arabidopsis seedlings.
“One of the reasons this has been forgotten is that peroxisomes in yeast and mammalian cells are smaller than the resolution of light,” Wright said. “With fluorescence microscopy, you could only ever see a dot. It’s just the limit that light can make.”
The peroxisomes he observed were up to 100 times larger. Scientists are not sure why peroxisomes get so big in Arabidopsis seedlings, but they do know that germinating Arabidopsis seeds get all their energy from the stored fat, until the leaves of the seedlings can begin to produce energy from photosynthesis. During germination, they are supported by countless tiny oil droplets, and their peroxisomes have to work overtime to process the oil. When they do, they get several times bigger than normal.
“The bright fluorescent proteins, in combination with the much larger peroxisomes in Arabidopsis, made this extremely noticeable and much easier to see,” Wright said.
But peroxisomes are also highly conserved, from plants to yeast to humans, and Bartel said there was some evidence to suggest that these structures could be general characteristics of peroxisomes.
“Peroxisomes are a basic organelle that has been with eukaryotes for a very long time, and there have been observations on eukaryotes, often especially mutants, where peroxisomes are either larger or less rich in protein, and therefore easier to visualize, ”told me. But people didn’t necessarily pay attention to these observations because the enlarged peroxisomes were the result of known mutations.
Researchers aren’t sure what the sub-departments are for, but Wright is speculating.
“When you talk about things like beta-oxidation or fat metabolism, you get to a point where the molecules don’t want to be in the water anymore,” Wright said. “When you think of a traditional type of biochemical reaction, we just have a substrate floating in the aquatic environment of a cell – light – and interacting with enzymes; it doesn’t work so well if you have something that doesn’t. doesn’t work. I don’t want to hang around in the water. “
“So if you use these membranes to solubilize water-insoluble metabolites and allow better access to lumenal enzymes, it may represent a general strategy to deal with this type of metabolism more effectively,” he said.
Bartel said the discovery also provides a new context for understanding peroxisomal disorders.
“This work could give us a way to understand some of the symptoms and potentially study the biochemistry that causes them,” she said.
New research into how a group of new organelle-based disorders affect cells
Zachary J. Wright et al, Peroxisomes form intralumenal vesicles with roles in fatty acid catabolism and protein compartmentalization in Arabidopsis, Nature communications (2020). DOI: 10.1038 / s41467-020-20099-y
Provided by Rice University
Quote: Hidden Structure Found in Essential Metabolic Machines (2020, December 4) Retrieved December 4, 2020 from https://phys.org/news/2020-12-hidden-essential-metabolic-machinery.html
This document is subject to copyright. Other than fair use for study or private research, no part may be reproduced without written permission. The content is provided for information only.
[ad_2]
Source link