[ad_1]
Increasingly influential, the cost-effective drug watchdog ICER does not believe that drugs approved for treating Duchenne muscular dystrophy have proven their value to patients. On Thursday, they used significantly higher price estimates for two therapies in their models, making them even less attractive – and acknowledged that the estimates used in their draft report in May were incorrect.
ICER's updated report on PTC Therapeutics Steroid $ PTCT Deflazacort and Sarepta $ SRPT Exondys 51 arrives before an expert meeting to discuss all of the evidence underlying the treatment of existing and future therapies. Duchenne muscular dystrophy (DMD). In their analysis, ICER used a price greater than $ 1 million a year for Exondys 51 and $ 81,400 for Emflaza – a former corticosteroid formerly imported by many overseas families at a cost of $ 50,000. about $ 1,000 a year.
In the United States, about 6,000 young boys suffer from muscle wasting syndrome caused by the absence of dystrophin, a protein that helps keep muscle cells intact. Symptoms tend to manifest themselves and progressively worsen between 3 and 5 years of age, usually requiring the patient to wheelchair in early adolescence. Finally, patients succumb to the disease at 30 years of age. Corticosteroids, which work by decreasing inflammation and limiting the activity of the immune system, are commonly used to treat DMD.
ICER evaluated the effectiveness, safety and cost-effectiveness of four treatments in its evidence report. He examined the steroids deflazacort (sold under the name of Emflaza) and prednisone – as well as the exon leap drugs: Explins approved (marketed as Exondys 51) and golodirsen. experimental (both of Sarepta), and each treatment is designed to treat subgroup of DMD patients). In concluding his May Preliminary Report, ICER reiterated that the evidence supporting each of the four treatments was lacking and that their impact on the patients was not clear.
In the evidence report released Thursday, which precedes an advisory committee meeting that will make its recommendations to the ICER on July 25, used higher annual cost estimates for Exondys 51 and Emflaza to perform various profitability calculations.
The dosage of the DMD drug is based on weight, which usually varies between patients. As in its May report, ICER used annual cost estimates for a 40-kg patient to calculate cost-effectiveness in its latest update, highlighting the controversy surrounding DMD drugs.
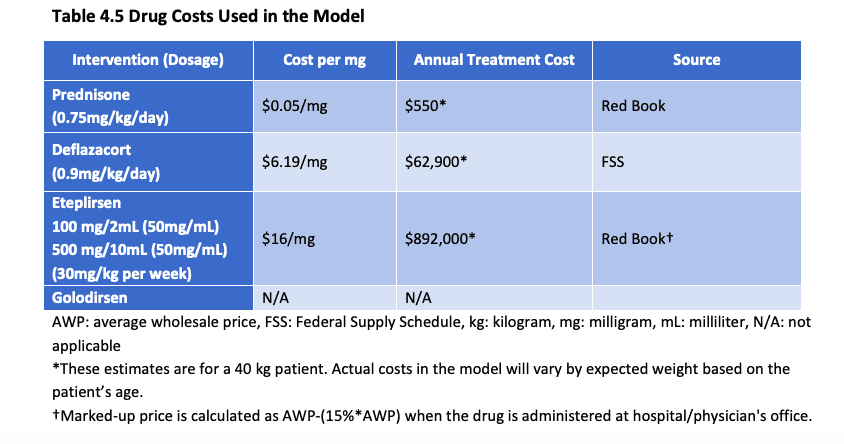
In the proof report, ICER presented this table:
Source: ICER, July 2019

Click on the image to see the enlarged version
In May, when ICER released its preliminary report, the non-profit organization presented this graph:
Source: ICER, May 2019
Click on the image to see the enlarged version
"We do not know what factors ICER used in their modeling. We have not increased the price of the drug since its launch and do not expect any price increase, "said a spokesman for Sarepta News on the end points.
PTC approved. Emflaza's prices and discounts have not changed, said a spokesman for News on the end points.
Later on Friday, a spokesman for ICER said the table in the previous draft report showed incorrect annual costs for Emflaza and Exondys 51. ICER's final report is expected to be released in August.
Social Image: Sarepta, AP Images
[ad_2]
Source link