
[ad_1]
It was the fastest vaccine development in history. But now the pharmaceutical companies are preparing to start over.
With the emergence of disturbing variants that could make Sars-Cov-2, the virus responsible for Covid-19, more transmissible, more deadly or more resistant to vaccines, drug makers who have responded in record time are rushing to ward off new threats.
Scientists are convinced they can answer. The question is: how fast?
Moderna – who, along with rivals such as BioNTech / Pfizer and the University of Oxford / AstraZeneca partnership developed their vaccines in less than 12 months – announced on Monday that it would begin human trials of a new designed booster to target strain 501.v2 first. identified in South Africa.
“We just want to be very careful because this virus is delicate, as we all learned the hard way last year,” Stéphane Bancel, CEO of Moderna, told the Financial Times. “We cannot stand still now. . . because it would take months to prepare the next one in case we needed it. “
Moderna decided to start a new trial after finding that its vaccine caused antibody levels six times lower for 501.v2 than for other strains already identified.
One of the reasons for this rush is that the more widespread the virus, the more likely it is to mutate.
Although data to date suggests that existing vaccines are generally effective against some of the more prevalent variants, including B.1.1.7 which has been responsible for a sharp increase in cases in the UK and the 501 variant. v2, no vaccine producer has published data on the disturbing P.1 variant that has been found in Brazil.
Data to date suggests that existing vaccines are effective against some of the more prevalent variants © Joseph Prezioso / AFP / Getty
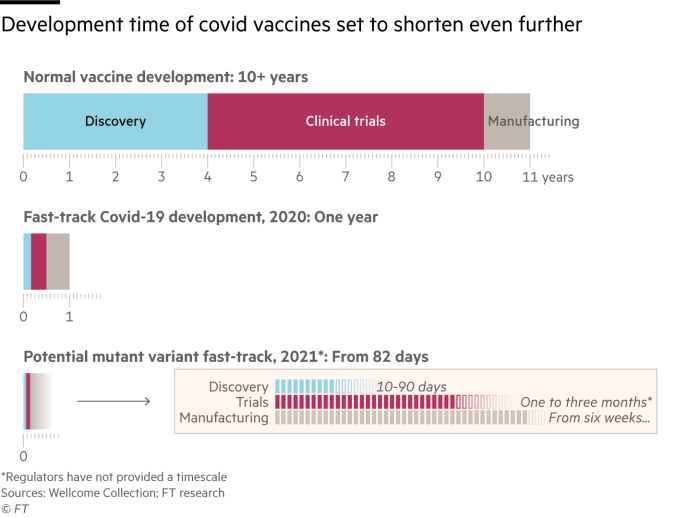
For pharmaceutical companies, saving as much time as possible on initial development, testing and manufacturing will be crucial if existing vaccines stop working against emerging strains.
“The vaccines used today are all based on the original Wuhan [viral DNA] and it’s likely that if there’s an impact on vaccines, it would be at all levels, ”said Dan Barouch, a Harvard researcher who helped design Johnson & Johnson’s vaccine candidate.
It is not about the technology behind the manufacture of the vaccine, he added. “It’s about when to pull the trigger to find out if you’re making a new vaccine.”
Preparation of vaccine prototypes and boosters
In anticipation of the worst, scientists worked on trial vaccines that could work against recent variants such as 501.v2 and P.1.
“As soon as these variants became apparent, we started working on vaccines targeting [them]Said Mene Pangalos, executive vice president of biopharmaceutical R&D at AstraZeneca.
He said it would take one to three months before the prototypes were ready and the goal was to have a candidate who would target “all the major variants in circulation.” Then the company would start clinical trials.
Nathalie Landry, executive vice president of scientific and medical affairs at Canadian pharmaceutical company Medicago, which is developing three vaccine candidates, said she was also working on prototypes. “I would expect every vaccine manufacturer to do the same.”
Ms Landry said it takes around 20 days to insert and grow the genetic sequence of the new variant in a plant culture to form virus-like particles. This then tricks the immune system into thinking it is encountering a viral particle.
Teams producing other types of vaccines said the process of making a new vaccine prototype would only take a few days.
She highlighted the annual flu shot, which is changed every year based on the most virulent strains in circulation. Since there are often several different strains, vaccine manufacturers produce four separate vaccines to be put into a single injection.
Preparing vaccine prototypes and boosters now will allow companies to move quickly to small-scale trials when new strains of the virus emerge.
With Sars-Cov-2, Ms Landry explained that if the World Health Organization or regulators determined that a previous strain was no longer circulating, “we would completely pivot manufacturing” but “if the recommendation is that we will have to add another strain, we would have to divide our production in two ”.
Existing technology can be used
Mr Bancel said Moderna went from seeing the virus sequence to shipping the first doses of its test vaccine in 42 days, but that “we could probably be faster on a new version, if necessary. “.
He told the FT that changing the manufacture would be “very easy” because almost everything in the formulation of the drug would remain the same. The only thing that is different is the genetic sequence, made up of “plasmid”, a small circular piece of DNA. Moderna plans to produce new plasmids for the 501.v2 variant and to store this ingredient in case it becomes necessary.
Stéphane Bancel, Managing Director of Moderna, said switching manufacturing would be “ very easy ” © Adam Glanzman / Bloomberg
German company BioNTech, which like Moderna has produced a new type of vaccine based on messenger RNA, said it could use existing technology to quickly produce a new vaccine against mutations in the virus.
“The beauty of mRNA technology is that we can directly start to design a vaccine that completely mimics this new mutation and we could make a new vaccine in six weeks,” said Ugur Sahin, chief executive of BioNTech.
AstraZeneca’s Pangalos also admitted that mRNA vaccines “probably have a 4-6 week benefit”.
Umesh Shaligram, director of research and development at the Serum Institute of India, the world’s largest vaccine maker, said “remaking the vaccine won’t take a long time.”
In partnership with five international pharmaceutical groups, including AstraZeneca and Novavax, the Serum Institute has committed to producing 1 billion doses of vaccine by 2021.
Dr Shaligram said the company has the technology to replace the crucial vaccine ingredient without changing any of the other components. Nonetheless, he admitted that building manufacturing capacity could take six months.
‘Transition studies’ could be essential
Discussions with regulators have resulted in loose commitments that compressed studies will be accepted as evidence of the safety and efficacy of new vaccine formulations. But there is still a debate about what exactly these trials would involve.
The UK Medicines and Health Products Regulatory Agency said it would likely require “bridging studies” – to bring together existing data on quality, safety and efficacy and information on the changed product. He said he would seek to conduct them “as soon as possible”.
Mr Bancel de Moderna pointed out that seasonal transition studies for the influenza vaccine – considered the best regulatory model for Covid-19 – typically involve a few thousand participants, rather than the tens of thousands required in preliminary studies .
Mr Pangalos said if the studies were only to demonstrate a robust immune response, they would likely only require 1,000 patients. For AstraZeneca, these trials could take up to three months, he added. “We would have the data by the end of the year in time for the next winter season, that would be the goal.”
For Covid-19 vaccines that have already been authorized, the United States Food and Drug Administration required an average of two months of follow-up data to monitor safety, which extended the time to market.
But Andrey Zarur, chief executive of GreenLight Biosciences, which is developing a Covid-19 mRNA vaccine, said the FDA had informed the company that it would not need to re-do safety testing if all of the vaccine’s ingredients were the same and only the viral sequence. has been changed.
“We have the assurance from the FDA that if we go with a DNA strain and then modify it, we wouldn’t need to start clinical trials at an early stage at all,” he said. .
The FDA said it had already “considered developing a potential pathway” for changes to Covid-19 vaccines and treatments.
Look for a holy grail vaccine
Several large laboratories are studying how the virus mutates in the face of threats, to try to anticipate the likely variants.
Others are researching a holy grail vaccine that would work against all coronaviruses, not just Sars-Cov-2.
Vir Biotechnology, a San Francisco-based infectious disease company, is working with GlaxoSmithKline on a universal vaccine.
“I think we have a good chance of finding [one] but it will take years, not months, ”said George Scangos, CEO of Vir Biotechnology.
Barney Graham, deputy director of the Vaccine Research Center at the US National Institutes of Health, said a universal vaccine was the ultimate goal, and he’s been working on since 2017. But there was little time for this guy. research during a pandemic.
Jesse Goodman, former chief scientist at the FDA and professor at Georgetown University, believes governments and regulators should push for such a vaccine and that studies of promising candidates should begin immediately.
“I don’t think it’s a question of whether one day we will have a different strain of coronavirus that has significant potential for evading vaccine immunity, but rather when,” he said. “You don’t want to wait for this to actually happen to sort out what you can do.”
Additional reporting by Stephanie Findlay
[ad_2]
Source link


