
[ad_1]
July 6: National alert to Japanese encephalitis issued
■ 7/6, alert to Japanese encephalitis issued

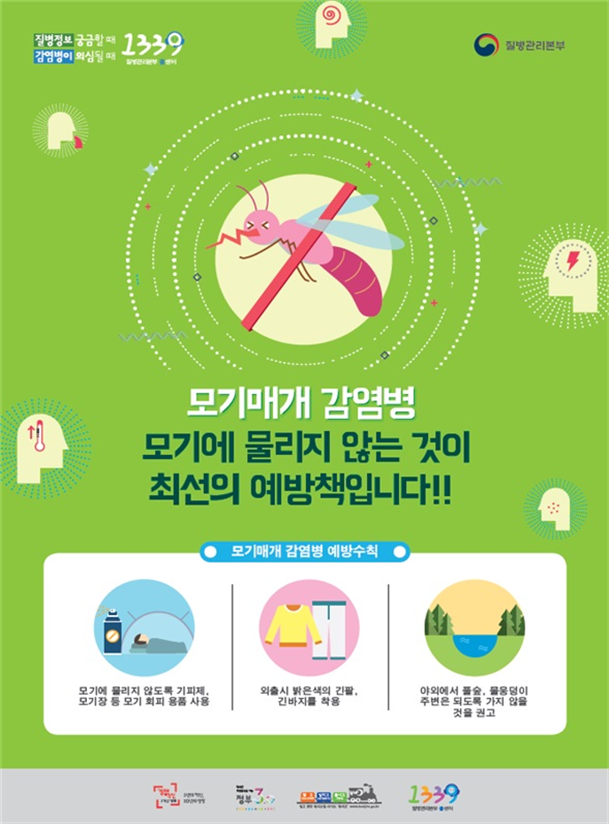
From July to the end of October, July 6 , the Center for Disease Control reported that a small red house mosquito, a Japanese encephalitis mosquito, had been found in the Chonnam area above the alarm emission criteria and had issued a national warning on Japanese encephalitis.
* An alert is issued when the number of small red mosquitoes among the average number of mosquitoes collected twice a week is greater than 500 and greater than 50% of the total mosquito density.
– Japanese encephalitis virus C is an infectious disease of the second group that causes acute nervous system symptoms due to the Japanese encephalitis virus that has pbaded through mosquitoes and it's spread in the blood. More than 99% of patients have mild symptoms with fever without symptoms, but some may progress to acute encephalitis, and about 30% may develop death if encephalitis develops. Even though recovered, one in three suffered from various neurological complications.
– This year's announcement was delayed by one week compared to last year (June 29, 2017), but only three or four years ago, when a warning was issued early August 5 days, August 6, 2015 and July 11, 2016). The period was accelerated by one month.1 In particular, an alarm was triggered in June for the first time in 20 years since 1997,
– From July to the end of October, the mosquitoes responsible for the disease are active, and 3 Japanese with Japanese encephalitis in the last 5 years were infected from June to September It is reported that more than 98% of patients between August and November started the first patient in the month.
– When Japanese encephalitis develops, there is no special treatment other than a conservative treatment.
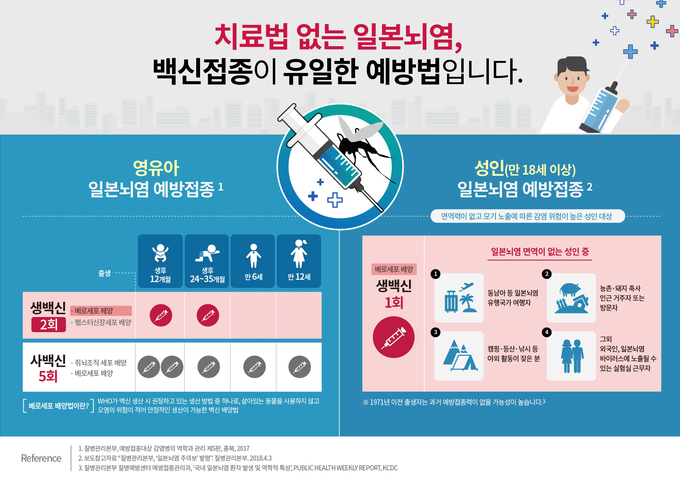
■ After the age of 12 months, infants and adults at high risk should be vaccinated in a timely manner
– Infants and Toddlers Vaccination is Possible from 12 months, which is a stone age.
– Vaccines that can be vaccinated in infants differ in the number of vaccinations and the schedule to follow. The live vaccine can be inoculated at the first dose after 12 months and at the second dose every 12-24 months. If the child had been vaccinated once in the past year, it would be possible to inoculate the vaccine this year, which will take place 12 months later. On the other hand, the vaccine is inoculated one to three times after the age of 12 to 35 months and once every 6 to 12 years, the vaccination is completed 5 times for 12 years. If the 1st or 2nd inoculation is done, the first inoculation will be completed by the first inoculation once this year, which is the point after 12 months, but defense immunity will be formed if additional vaccination does not occur. is not missed at 6 years old and 12 years old.
– Vaccinations are recommended for adults who plan to travel to countries where Japanese encephalitis mosquitoes are common or who live near paddy fields and Japanese encephalitis outbreaks such as Asia Southeast3. If you were born before 1971, it is likely that you will not be vaccinated, so vaccination is no longer necessary. [19659000]
– The "Emo" of Sanofi Pasteur is recommended by the World Health Organization (WHO) C & # 39; is a living blood culture of the Japanese encephalitis BERO cell developed by the cell culture method. In 2015, the only live vaccine that can be vaccinated by KFDA infants and adults is registered and can currently be vaccinated. (19659007) – The BERO method is one of the vaccine production methods recommended by the World Health Organization (WHO): it does not use live animal cells , the risk of contamination is low and stable production is possible. Immuno does not contain ingredients that may cause allergic reactions such as mercury preservatives, antibiotics and gelatin and has been approved by the World Health Organization (WHO) for preliminary screening of quality, safety and effectiveness.
– Infants and toddlers can be completely inoculated in two years with a single dose of auricide (two doses 12 to 24 months after the first dose after the age of 12 months ). (In Korean clinical trials in healthy infants aged 12 to 24 months after birth, 100% serum anti-erectile levels were confirmed 28 days after first vaccination and healthy children between 12 and 24 years of age. month after the first inoculation.In the clinical trial, the vaccination rate was 100% after 15 days of inoculation and 15% of patients had a high serum antibody level and 14 ~ 19659008 – Defensive Immune Formulas (In clinical trials conducted in Australia and the United States in adults aged 18 years and older, it showed a rapid and high preventive effect 14 days after inoculation after Inoculation 14 days after inoculation, 93.6% and 99.1%
1 Press Release "Headquarters of Disease Control," warning of Japanese encephalitis "delivered." Mala control headquarters dies. [Référence] "Headquarters of Disease Control," Warning on Japanese encephalitis "published by the Ministry of Health and Welfare. Headquarters of disease control. "Report of the Japanese Center for Disease Control and Prevention," Japanese Encephalitis Alert. "Headquarters Disease Control 2017.6.29
5 Press Release." Japanese Encephalitis Warning issued to the Nationwide, Japanese encephalitis – mosquito-mediated more than 50% of the total mosquito density. "Headquarters Disease Control 2012.07.19
7 Press Release" National Immunization! Protecting the Republic of Korea Against Infectious Diseases "Headquarters for Disease Control
9 Tian Chen et al.,
8 Development and Epidemiological Characteristics of Japanese Patients with Encephalitis Department of Immunization Management, Center for Disease Control, Investigation and Application Progress of Vero Cell Serum-fee Culture, International Journal of Biology, p.41
11 Australian Public Assessment Report Vaccine against Japanese encephalitis (live, attenuated) Imojev Sanofi-Aventis Australia Pty Ltd.
14 Product Monograph Imojev, p.28
12 Australian Consumer Medicine Information, V1.1August 2012
13 Letter of Acceptance from WHO 2014-433 of 17 September 2014
19659006] 15A Randomized Study of Immunogenicity and the security of the Japanese chimeric encephalitis vaccine against the virus (JE-CV) in comparison with the SA14-14-2 vaccine in children in Korea, Dong Kim SOO, Alian Bouckenooghe, 2014 p.17
17 J. Torresi et al. Vaccine 2010; 28: 7993-8000
Source link