
[ad_1]
Jongjongwon Jangwon Biospectator
& # 39; Molecular Therapy-Nucleic Acids & # 39; released .. HDAC4 and microRNA (miR) -206 acting on the improvement of the phenomenon of atrophy & # 39; .. US phase 2 preparations
Viromed The researchers found that VM202, a plasmid-based gene therapy drug that contains a human hepatocyte growth factor (HGF) gene, has been shown to be effective in the treatment of amyotrophic lateral sclerosis (ALS). Demonstrate that VM202 is acting on HDAC4 and microRNA (miR) -206 to improve muscle atrophy. ViroMed has completed Phase 1 of US Phase 1 of ALS Therapeutics and is preparing for Phase 2.
ViroMed announced on May 30 that VM202 has scientifically proven that it can relieve neurological muscle atrophy and improve the symptoms.
This study investigated the therapeutic effect of VM202 in a model of muscle atrophy caused by peripheral nerve injury, and molecular biology. Specifically, we studied changes in the expression of hepatocyte growth factor (HGF) and its receptor c-met when muscle atrophy was induced by peripheral nerve injury. (2)

As a result, the expression of hepatocyte and c-met growth factor was greatly increased when muscle atrophy was induced by cutting sciatic nerve from the rat model. . The sciatic nerve is a large peripheral nerve that pbades above the knee and at the back of the thigh and is responsible for the external sensation of the legs and the back of the calf under the ankle and knee
. Consequently. This implies that HGF and c-met act on the process of atrophy caused by peripheral nerve injury. In addition, the enzyme HDAC4 and microRNA (miR) -206 play a major role in the process of atrophy, and HGF / c-met can regulate this protein and microRNA.
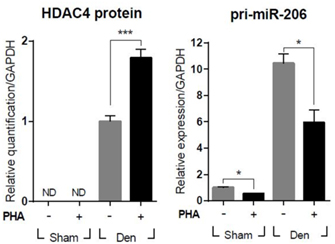
In addition, when VM202 was injected, it was confirmed that atrophy was attenuated and histological improvement was observed.
Kim Sun-Young, president of the Research Institute of the Korea Cancer Center, said, "This paper is based on the results showing that VM202 has been shown to be effective in treating the SLA.1 on the effect of treatment
ViroMed has completed Phase I clinical trials in the United States for amyotrophic lateral sclerosis disease.
In addition, VM202 has been designated as a orphan drug and FAST TRACK by the US FDA and has been approved by the US FDA for Phase 2 clinical trials.

] (Function, (d, s, id) {} ( 19659012)
var js, fjs = d.getElementsByTagName (s) [0];
if (d.getElementById (id)) returns;
js = d.createElement (s); js.id = id;
js.src = "http://connect.facebook.net/en_US/sdk.js#xfbml=1&version=v2.6&appId=282929402052063";
fjs.parentNode.insertBefore (js, fjs);
} (document, 'script', 'facebook-jssdk'));
[ad_2]
Source link
Tags diseasequot Gehrig39s Lou quotVM202 treatment Vyromed