[ad_1]
By Eduardo Simões
SAO PAULO (Reuters) – A coronavirus vaccine developed by Chinese Sinovac Biotech was 78% effective in an advanced stage Brazilian trial and completely prevented severe cases of COVID-19, researchers said Thursday, although ‘A lack of data details has prompted calls for more transparency.
The announced effectiveness, closely watched by developing countries that rely on the vaccine to start mass vaccines to help end a raging pandemic, was lower than preliminary findings by Turkish researchers and lacked detailed data provided. on American and European vaccines.
The director of the Brazilian biomedical center Butantan, a research and production partner of Sinovac, said detailed results were being submitted to health regulator Anvisa as part of a request for emergency use of the vaccine.
“One thing is a presentation at a press conference. It’s another thing to get the data and analyze it, which Anvisa will do,” said Cristina Bonorino, who sits on the scientific committee of the Brazilian Society of Immunology. . “If that’s what they say, it’s a great result,” she added.
Brazil and Indonesia, which respectively have the most COVID-19 cases in Latin America and Southeast Asia, are preparing to roll out the vaccine, called CoronaVac, this month. Turkey, Chile, Singapore, Ukraine and Thailand also have supply agreements with Sinovac.
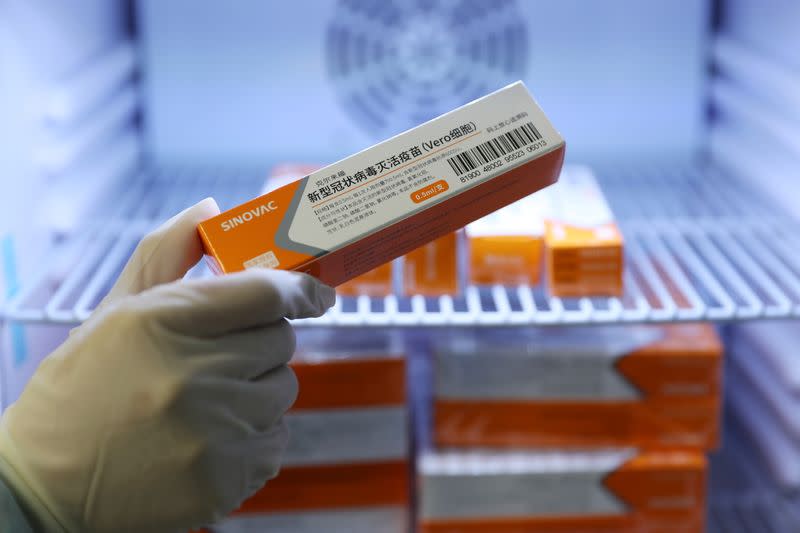
Although CoronaVac’s efficacy is lower than the 95% vaccine success rate of Moderna Inc or Pfizer Inc with its partner BioNTech SE, it is easier to transport and can be stored at normal refrigerator temperatures.
The 78% efficacy rate is also well above the benchmark of 50% to 60% set by global health authorities for vaccines in development at the start of the pandemic, given the urgent need.
Moderna and Pfizer / BioNTech released detailed results from late-stage trials last year, before receiving emergency use approvals in the United States and elsewhere.
Butantan director Dimas Covas told a press conference that full CoronaVac data will be released in an unspecified scientific publication, but did not provide a timeline.
Pressed by reporters, Covas said there had been 218 cases of COVID-19 in the trial of 13,000 volunteers. Just over 160 of those cases occurred in participants who received a placebo and the rest in vaccinated volunteers, he said.
The CoronaVac trial in Brazil included elderly volunteers, unlike other vaccine studies.
Covas said CoronaVac completely avoided severe cases of COVID-19 among the vaccinated group, including the elderly. None of those who received the vaccine are sick enough to require hospitalization, he added.
SOME DETAILS
The fragmentary disclosure of the results of the global CoronaVac studies has raised concerns about the transparency of the trials.
Dr Gregory Poland, a virologist and vaccine researcher at the Mayo Clinic in Rochester, Minnesota, complained that Thursday’s news from Brazil was “devoid of any real details.”
“I want to see the data. I want to understand the methods and what has been done. And I want to see a real peer-reviewed publication, not a press release,” he said.
The partial disclosure of Butantan, who had delayed his announcement three times, citing obligations to Sinovac, added to skepticism about the Chinese vaccine in Brazil. Nearly half of Brazilians said they would not take a COVID-19 vaccine developed by China, according to a December poll.
Brazilian President Jair Bolsonaro has expressed contempt for the Sinovac vaccine, citing doubts about its “origin”. He traded beards with his political rival João Doria, the governor of Sao Paulo, who funds the trials and production of the shot.
Brazil has the second deadliest epidemic in the world after the United States with nearly 200,000 coronavirus-related deaths, and aims to vaccinate 51 million people, or about a quarter of its population, in the first half of 2021, although vaccinations have not yet started.
Doria recalled that Sao Paulo, the richest and most populous state in the country, is expected to start vaccinations on January 25.
Based on traditional vaccine technology using an inactivated coronavirus to trigger an immune response, CoronaVac can be stored at temperatures 2 to 8 degrees Celsius (36 ° -46 ° F) and can remain stable for up to three years.
The vaccines offered by Pfizer / BioNTech and Moderna use new synthetic messenger RNA (mRNA) technology, requiring much cooler temperatures for shipping and storage. Pfizer / BioNTech vaccine must be stored at a subarctic temperature, making it an ineffective option for poor countries and regions without the required cold storage equipment.
(Reporting by Eduardo Simões Additional reporting by Julie Steenhuysen, Ana Mano, Anthony Boadle and Beijing Newsroom, edited by Brad Haynes, Miyoung Kim and Bill Berkrot)
[ad_2]
Source link