
[ad_1]
A scientist modified the DNA of infants to be immune to the AIDS virus. This is the latest controversy in genetic publishing. CRISPR has brought opportunities never explored. They are promising. But dangerous.
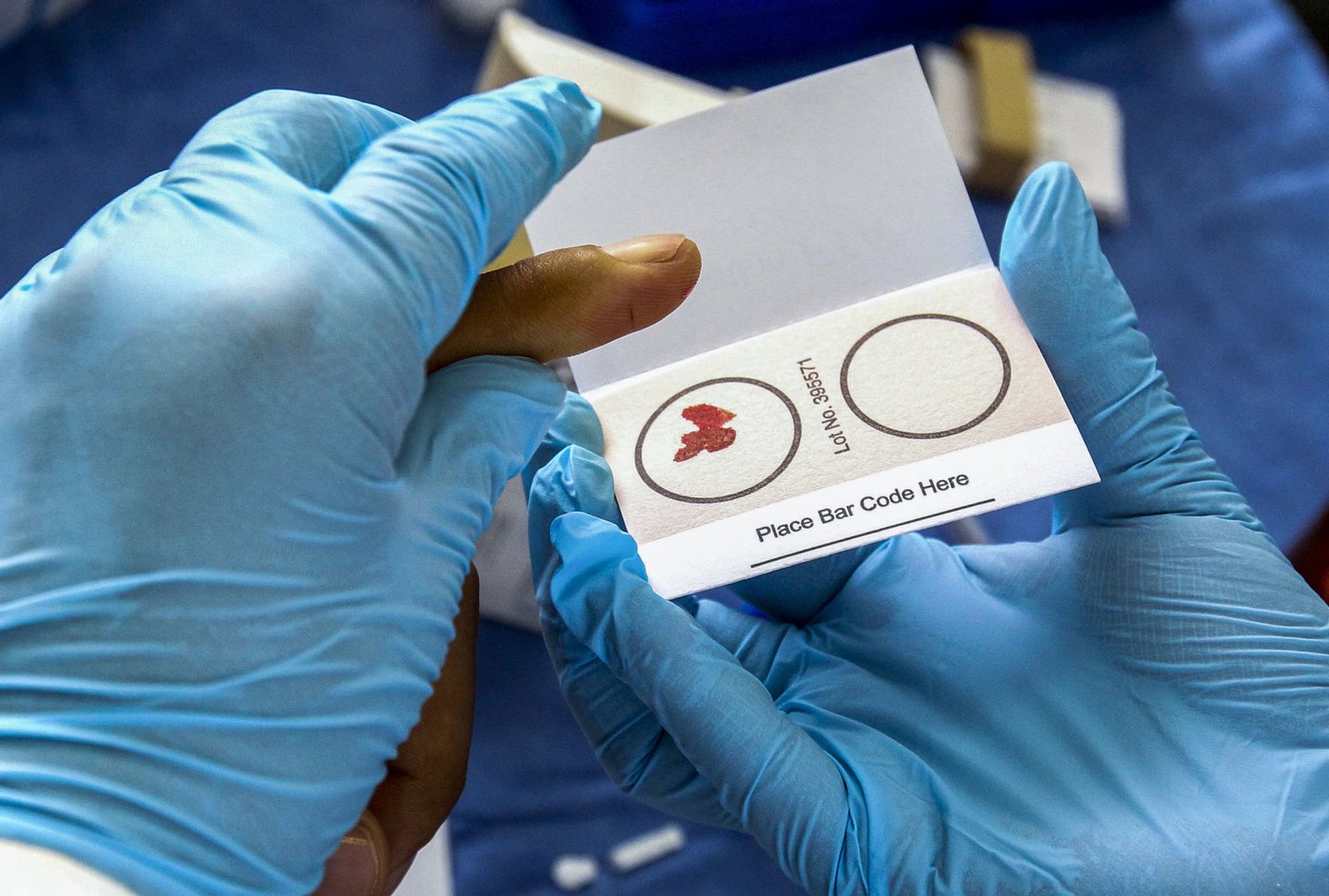
He Jiankui did not just want to be first. I wanted to be an example. What you did is done. And "society will decide what to do next".
The Chinese scientist took over Monday with the help of a powerful genetic tool called CRISPR-Cas9. It's with her that she has altered the genome of two twin babies during a couple's fertility treatments. It was very simple: first inject the tool into the embryos; then found a very specific part of his DNA; and then off. That's it. Nine months later, two girls were born, one of them with a genetic trait that almost no one possesses: the ability to resist future infections of the HIV virus, the AIDS virus.
Half of the scientific community was skeptical as to the allegations of He Jiankui. The study of the Chinese researcher's experiments has not yet been published in any scientific journal and has not yet been examined by other scientists. But then, He Jiankui spoke about this subject at the Second International Summit on the Publishing of Human Genetic Resources and the certainties became real: "We are sure. He made a pretty impressive presentation on the extensive research and He had done extensive work on human and animal embryos "said Helen O'Neill, CRISPR expert, to New Scientist. "It was later during the questioning session that we learned the juiciest things that people wanted to know, little by little we had more and more shocking information. 39, he himself partially funded the research, for example.Nearly by chance, when asked if there was another pregnancy, he replied: H Hmm …. yes, there is & # 39; . "
The other half of the scientific community was in shock. Firstly, "if something goes wrong, nothing can be stopped": "We can not know what effect it will have on the next generation." "He was transparent, he would have more confidence that" He has been doing so, but he has gone unnoticed for months and is on leave without pay, she does it alone, "said specialist Helen O'Neill. And second, because "the gene you chose was terrible": "There is an almost damaging layer for the mutation of a gene that makes it vulnerable to HIV." a potentially fatal disorder that had no other treatment, people would have found all this more justifiable. The impression within the scientific community is that it was the most simpler, because a lot of research has been done [sobre este gene] "she told New Scientist.
The steps in the history of genetic modification have been carefully specified. . We know very little about the consequences of the most daring changes we make to human DNA. Kiran Musunuru, a specialist at the University of Pennsylvania, told The Associated Press that He Jiankui's experiments were "unconscious, human experiences not morally and ethically defensible".
It is sufficient that the change operated with CRISPR is manufactured in a gene more than the way for the life of a person to be condemned. And even if it's healthy, no one knows what consequences it might have for future generations. Some say that we should not risk when we talk about human life. That's the case of Eric Topol, head of the Scripps Research Translational Institute: "It's too early. We're dealing with the way a human being works." Others say the sacrifice pays off so it provides a life-saving tool: "It's perfectly justifiable," said George Church, geneticist at the University of Toronto. 39, Harvard University.
A change is needed with CRISPR. gene on the side for the life of the person to be condemned. And even if it's healthy, no one knows what consequences it could have for future generations. Some say that we should not risk when we talk about human life.
Thanks to CRISPR, we are able to eliminate unwanted parts of genetic information and replace them with more convenient parts. Thanks to this, we have been able to avoid deadly genetic diseases, create pork organs that can be used by humans and attack cancer from the inside. This is the promise of a better future and the genetic aspect. But there is the other side of the coin: if you fall into the wrong hands, CRISPR can also be used to introduce deadly diseases to the human race or to catalog babies, with features chosen on a sudden basis. head. Which side should be the balance?
A story that has roots in Spain
The history of human genetics begins on the beaches located near the Mediterranean port of Santa Pola, Spain. Francisco Mojica grew up near the Costa Blanca. At the age of 26, while she was preparing a PhD at the University of Alicante, she began studying an isolated microbe from the Santa Pola saltworks, which produce four thousand tons of salt a day. It was a special microbe: Haloferax mediterranei was much more resistant to these saline than any other. But it was special, especially because he had in his genes a tool both promising and dangerous for humans.
In the beginning, nobody wanted to finance Francisco Mojica's research. When asked why he wanted to study this microbe, Mojica could never explain: "I wanted to know, know, to increase knowledge." When I asked for money for my investigations, nobody did not give me because no one wanted to know what I was looking for.
Coarse mistake because, with this microbe, Francisco Mojica discovered a new genetic publishing technology having a millionaire value that promises to revolutionize human life as we know it, for the better and for the worse or the evil.It is CRISPR, a kind of cut-and-paste which, in good hands, can solve the most serious hereditary diseases
CRISPR is an acronym for "grouped and regularly spaced palindromic repetitions", a very simple and powerful tool that allows scientists to modify minimally invasively the DNA of a living being and modify l programmed functions for certain genes ". CRISPR technology allows scientists to make DNA changes in cells that can help us cure genetic diseases, "said Jennifer Doudna, one of the most prominent scientists in the field, at a TED conference
bacteria have to cope with viruses in the environment they live in and viral infections can be considered a ticking time bomb, "he compared to biochemistry American: "A bacterium has a few minutes to deactivate the bomb before it is destroyed .Therefore, many bacteria have in their cells an adaptive immune system called CRISPR that allows them to detect and destroying viral DNA . "
All of this is done in stages. When the bacteria is attacked, it stores the genetic information of the virus in specific DNA sites called CRISPR. If the virus attacks the bacteria again, it recognizes the genetic information of the virus and creates molecules called RNAs, which are perfect copies of this information. Then, these RNA molecules bind to a protein called Cas9 – an enzyme that acts like a pair of molecular scissors and removes fragments of genetic information – and forms a complex that functions as a sentinel. This is CRISPR-Cas9, whose function is to search all the DNA of the cell to find the site corresponding to the RNA sequences. When it finds these sites, the complex binds to the DNA and allows the Cas9 protein to accurately cut viral DNA.
A simple copy / paste genetic.
The 4-Step Recipe for Genetic Publishing [19659026] But why can this be useful for humans? Because scientists can isolate the tool CRISPR-Cas9 and then manipulate all the information in order to replace or correct genetic mutations, says José Carlos Bessa of the Institute of Molecular and Cell Biology from the University of Porto to the observer. "Viruses are capable of introducing things into the cells, in which case they can introduce genetic information.How can we control the genetic information carried by a virus? it contains the desired information. "
The recipe for inserting this tool into a cell goes through four steps. The first is to introduce a virus into the cell with the genetic mutation that counts. And this can be done in two ways: by transfection, which mimics an infection; or by a micro-needle in case of very large cells. The second step is to "give a genetic address" which tells CRISPR where the DNA error is. Then, the Cas9 protein uses this sequence to look for the error indicated in CRISPR. The fourth step is to eliminate what is wrong
But why can this be useful to humans? Because scientists can isolate the CRISPR-Cas9 tool, and then manipulate all the information to replace or correct genetic mutations, says José Carlos Bessa of the Institute of Molecular and Cell Biology of the University of Porto to the Observer.
This happens because, as in bacteria, CRISPR uses an RNA molecule that serves as a guide, says observer José Carlos Bessa. This molecule has a length of 105 bases, 20 of which combine with the wrong sequence of the gene, so it is sufficient to change these bases so that the Cas9 protein can be transferred to any DNA site.
Two things can happen then. : The protein Cas9 can suppress the genetic error to eliminate or modify the bad material by introducing a new information, this time correct, in the white. In the latter case, the guide wire RNA brings the Cas9 protein to where it needs to be modified, and then introduces a correct model in the white. This type of "RNA" looks like a blind dog, "compares another investigator with whom the observer spoke, Pedro Dias Ramos.
What is CRISPR Special?
The idea of manipulating genetic material with the help of tools recognize specific regions of DNA and generate small mutations "is not extremely Innovative " explains to the Observer researcher José Carlos Bessa of the Institute of Molecular and Cell Biology of the University of Porto. Since 2009, scientists have been using enzymes called TALEN to cut specific DNA fragments. and proteins called zinc fingers, which also have the ability to bind to a strand of DNA
But CRISPR has an advantage over these tools: is much simpler and faster to use . According to Feng Zhang, a Broad Institute biochemist, "CRISPR-Cas9 has proven to be an effective and customizable alternative to other genome editing tools." "As the CRISPR-Cas9 system itself is capable of cutting portions of DNA, CRISPRs do not have to be badociated with separate cleavage enzymes, unlike other tools," says the scientist.
The idea of manipulating genetic material with the help of tools that recognize specific regions of DNA and generate small mutations "is not extremely innovative". Since 2009, scientists have been using enzymes called TALEN to cut specific DNA fragments. and proteins called zinc fingers that also have the ability to bind to a strand of DNA.
The problem with TALEN and the zinc fingers is that they "require months of validation" says José Carlos Bessa: "The creative process has two parts: one is the development of 39; an enzyme that recognizes a specific sequence of genetic material and the other is the creation of a protein that will trigger mutagenesis, that is to say modify the genetic information for create a mutation. "
The two parts are paired, but" it's such a specific process that every step has to be checked and it takes longer. " On the other hand, when working with CRISPR, this recipe is no longer needed because the tool already has two parts: one is CRISPR itself and the other, Cas9, the protein able to cut specific sites in the chain. DNA for which it is programmed in the laboratory.
We can then talk about the three main advantages of CRISPR over other existing tools to date, according to the Jackson lab. First of all, simplicity, because "RNA guides can be designed easily and inexpensively to specifically target any genome sequence". Second, the effectiveness: "Changes can be introduced by direct injection of RNA encoding the protein Cas and guide RNA in the development of rat embryos." And third, the amount of changes that can be made at once: "Multiple mutations can be introduced into multiple genes at once, injecting them with multiple RNA guides."
CRISPR has been described as revolutionary. – perhaps even the greatest breakthrough for the progress of medicine during this decade. Since the 1970s, it seems that all essential stages of health development occur every ten years. The first were the restriction enzymes, which cut the DNA molecule through the recognition of specific sequences and led to the development of new drugs and new research tools. Then, the polymerase chain reaction, a technique that replicates millions of copies of a specific DNA sequence. Then we were able to sequence the human genome with a rhythm and precision never achieved before. There are about 6,000 hereditary diseases in the world and 95% have no proven treatment. In addition, it is estimated that more than 10,000 human diseases result from the mutation of a single gene. But it is a reality about to change: if diseases are expressed by a single gene, scientists can easily use CRISPR to identify the wrong gene and remove it from the pathway or replace it with healthy genetic information. correct With this, a person with muscular dystrophy, cystic fibrosis or a form of blindness called Leber's optic neuropathy could be cured, for example
. We have already seen this happen. In the summer of 2017, a team from the Center for Embryonic Cells and Gene Therapy announced that it had eliminated heart disease, hypertrophic cardiomyopathy, in human embryos . This common and serious condition causes sudden death, especially in athletes and youth, and affects one in 500 people. Hypertrophic cardiomyopathy is caused by an error in the information contained in the MYBPC3 gene. To correct this gene, scientists used … CRISPR
In the summer of 2017, a team from the Embryonic Cell and Gene Therapy Center announced that it had eliminated heart disease, cardiomyopathy hypertrophic, in human embryos. This common and severe disease causes sudden death, especially in athletes and youth, and affects one in 500 people. To correct this gene, scientists used CRISPR.
Scientists injected eggs donated by healthy women with sperm from a sick man and CRISPR. This editing tool included such guides to RNA, the DNA-like acid responsible for synthesizing cellular proteins, whose function was to find the exact location where the genes had to be corrected. They also took the Cas9 enzyme to work as scissors and cut the defective part of the DNA. When this part was cut off, the correct information stored in CRISPR was pasted into the DNA and the genetic material was perfectly healthy.
Of the 58 embryos resulting from this in vitro pioneer 42 were completely free from the gene mutation at the origin of hypertrophic cardiomyopathy: the success of the technique was 72% . All became fully viable: they carried no genetic disease and could not transmit it to their offspring either. Thirteen of the embryos did not have any mutations, but not all cells.
Another great promise of CRISPR is the ability to rehabilitate organs of other animals so that they can be used by humans. Pork organs, for example, are very similar in size and anatomy to humans, but it is not possible to transpatate them for two reasons: first, because pigs can be infected with very dangerous viruses, such as hepatitis E; and second, because pigs have genetic information about viruses in DNA, which varies from generation to generation.
To solve the first problem, there are already effective vaccines on the market. To solve the second problem, we now have CRISPR. Also in the summer of 2017, a team of scientists from Harvard University and the eGenesis Society announced the birth of piglets whose genetic information from viruses had been deactivated. As in the process of selecting Dolly ewes, CRISPR was introduced into sow eggs with sperm. The embryos obtained were then placed in the uterus of other sows. Piglets born three months later were free of virus genes. In theory, their bodies could be used in humans.
A team of scientists from Harvard University and eGenesis announce the birth of piglets whose genetic information from viruses has been disabled. As in the process of selecting Dolly ewes, CRISPR was introduced into sow eggs with sperm. The embryos obtained were then placed in the uterus of other sows. Piglets born three months later were free of virus genes. In theory, their organs could be used in humans.
3 – Fight Against Cancer
The fight against cancer is another front in which CRISPR can enter. This application was actually the first one where CRISPR was injected directly into adult humans – and not to animals or embryos, as is usually the case in the test phases. In this case, the patient was suffering from lung cancer. Therefore, a Chinese team isolated immune system cells from a blood sample of a patient and injected them with CRISPR.
Already in the cells, CRISPR was addressing the gene encoding a protein called PD-1 that normally slows the immune system, preventing the body from fighting cancer and giving it space to grow and spread. Then, cells without the PD-1 protein were reinserted into the patient. It is too early to draw conclusions about the results of this experiment, but we hope to see the immune system attack lung cancer as it has never been before.
Still in the field of cancer control, a team from the University of Pittsburgh was able to lead CRISPR up to the "command center" of a cancer that l & # 39; 39 stopped growing and increased the average life expectancy of the mice used in the experiment. In this case, CRISPR has been introduced into the fusion genes, which occurs when two genes combine to form a hybrid gene producing abnormal proteins that cause cancer or aggravate an existing protein.
Moreover, the tool was still able to strengthen itself. the immune system to fight against cancers such as prostate, liver, lung or ovarian cancers. If in rats without CRISPR, cancer tumors increased nearly 40-fold and metastases spread, causing the death of all animals by the end of the study, in those with had received CRISPR, tumor size was reduced by up to 30%. and no metastasis has spread.
Another interesting novelty is that which arrived in May of last year when researchers from Temple University and the University of Pittsburgh managed to eliminate the material. genome of the AIDS virus derived from the genome of animals of three different species to ensure that the infectious agent ceases to replicate in the body of this living being. The scientists' strategy has even been successful in human immune system cells transplanted into laboratory mice.
Mosquitoes do the same with mosquitoes to prevent them from infecting the population with diseases such as malaria which kills 500,000 people a year. In the laboratory, it has already been possible to introduce CRISPR into a mosquito in order to replace its genetic information with another that immunizes it against the malaria parasite. Thus, the mosquito no longer transmits the disease to a person if it stings.
Mosquitoes do the same to avoid infecting the population with diseases such as malaria, which kills 500,000 people a year. In the laboratory, it has already been possible to introduce CRISPR into a mosquito in order to replace its genetic information with another that immunizes it against the malaria parasite. Thus, the mosquito no longer transmits the disease to a person if it stings.
But that is not enough: genetic modifications of this nature are generally inherited only half of the descendants of this mosquito and a quarter of their descendants. CRISPR also resolved this obstacle last year. According to Bloomberg, a team of scientists would have been able to alter the genetics of a mosquito so that any genetic change would be inherited more likely from subsequent generations. Only at the time of mating, almost 100% of the offspring of a mosquito modified with CRISPR can become immune to the malaria parasite.
At a time when the World Health Organization warns that "antibiotic resistance leads to higher medical costs, prolonged hospital stays and increased mortality" CRISPR can help fight bacteria that have become resistant to antibiotics prescribed by doctors and cause infections that are harder to treat. How to order them to commit suicide.
That's what Jan-Peter Van Pijkeren, scientist at the University of Wisconsin-Madison, is all about. According to an explanation given to the MIT Technology Review, the idea of Van Pijkeren is to develop in the laboratory viruses that infect only bacteria (phages) and that can carry CRISPR with a specific message: tell the bacteria to self-destruct. To introduce it into the person infected with super-bacteria, the phage would be mixed with other inoculated bacteria in tablet form .
The idea of Van Pijkeren is to develop in the laboratory viruses that infect only bacteria (phages) and can carry CRISPR with a specific message: that bacteria self-destruct. To introduce it into the person infected with the super-bacterium, the phages would be mixed with other harmless bacteria in tablet form.
6 – Improving crops
It is in the field of nutrition that CRISPR can become more common. Parce que dans ce domaine, les changements génétiques ne sont plus nouveaux : depuis les années 1990, nous avons mangé du fromage avec de la chymosine au lieu de la présure et des tomates transgéniques qui mûrissent plus lentement après la récolte.
fabriqué fondamentalement avec trois instruments: biolistique, qui tire quelques balles en métal recouvertes de fractions d’ADN qui entreront dans la composition du matériel génétique de la cellule cible; l'électroporation, qui utilise un champ électrique pour affaiblir les membranes cellulaires et rendre les produits chimiques plus réceptifs; et le système TALEN, qui utilise des protéines pour modifier des gènes spécifiques
CRISPR promet d'être moins cher et plus rapide dans ce processus. Au lieu de coûter cinq mille euros – le prix pratiqué actuellement -, les modifications génétiques ne coûteront que 50 dollars . De plus, grâce à cet outil, les agriculteurs pourraient rendre les aliments plus résistants aux maladies afin d'accroître leur capacité de production.
Un jour, un scientifique très important s'est adressé à Jennifer Doudna et lui a dit: "J'ai quelqu'un d'autre très puissant avec moi que je veux que vous sachiez et je veux que vous expliquiez comment cette technologie fonctionne. " Jennifer a dit oui, mais a été horrifiée lorsqu'elle est entrée dans la pièce et a réalisé qui l'attendait: Adolf Hitler. Hitler, a-t-elle dit, était badise sur un bureau, dos à Jennifer, " avec un visage ressemblant à un museau de cochon." Puis il se retourna, posa son stylo et dit: "Je veux comprendre les utilisations et les implications de cette technologie incroyable."
Ensuite, Jennifer s'est réveillée. C’était un cauchemar auquel s’ajoutait l’une de celles qui tourmentaient Jennifer depuis qu’elle et Emmanuelle Charpentier avaient proposé d’utiliser CRISPR pour éditer et reprogrammer des gènes, bien que cette découverte fût l’une des plus importantes de l’histoire de biologie. "Je me suis réveillé avec des sueurs froides. Suponho que se alguém como Hitler tivesse acesso a isto só podemos imaginar o tipo de usos horríveis que o CRISPR poderia ter“, contou ela no livro “GMO Sapiens: The Life Changing Science of Designer Babies” de Paul Knoepfler.
1 – Criar bebés à medida
Uma das maiores preocupações éticas que os cientistas levantaram no que toca à edição genética em geral e ao CRISPR-Cas9 em particular são os chamados “designer babies”. Esse é o nome dado aos embriões humanos que podem ser geneticamente modificados para que tenham as características que os pais ou os cientistas pretendam. E não estamos apenas a falar da eliminação de doenças graves que possam afetar a qualidade da vida daquela pessoa, mas sim de características físicas, algumas por mero capricho.
Tecnologias como o CRISPR podem, a longo prazo, permitir criar bebés como se estivés semos perante um catálogo. Isso é possível em teoria, mas nem tudo pode ser alterado ainda. Anna Olsson, investigadora do Instituto para a Biologia Celular e Molecular que trabalha na área de pesquisa responsável da edição genética, diz que "isso abre uma grande discussão porque para editar o genoma precisamos de saber que genes determinam determinadas características".
Tecnologias como o CRISPR podem, a longo prazo, permitir criar bebés como se estivéssemos perante um catálogo: escolher olhos castanhos em vez de azuis ou cabelo escuro em vez de claro. Isso é possível em teoria, mas nem tudo pode ser alterado ainda. Anna Olsson, investigadora do Instituto para a Biologia Celular e Molecular que trabalha na área de pesquisa responsável da edição genética, diz que “isso abre uma grande discussão porque para editar o genoma precisamos de saber que genes determinam determinadas características“.
2 – A muita falta de informação que ainda existe
Anna Olsoon explicou ao Observador que, se algumas características que temos dependem da informação codificada num só gene, outras características dependem do que está previsto em vários genes, alguns deles em coordenação. Nesse caso, o CRISPR pode não ser eficaz o suficiente para fazer alterações profundas até porque “ainda não sabemos por completo como funciona o genoma humano“: “É verdade que o genoma humano está todo sequenciado, mas a forma como se regula não é conhecida“, explica Pedro Dias Ramos, doutorando na Universidade do Porto cujo trabalho de investigação se centra na edição genética com o CRISPR.
O badunto não parece tão distante badim para a Organização das Nações Unidas para a Educação, a Ciência e a Cultura (UNESCO), que pediu “a proibição da edição do ADN humano para evitar adulterações antiéticas com características hereditárias” depois de uma reunião em Paris que juntou cientistas, filósofos, advogados, políticos e especialistas independentes do Comité Internacional de Bioética.
Todos concordam que “a terapia genética poderia ser um divisor de águas na história da medicina e na edição do genoma” mas que é “sem dúvida, um dos empreendimentos mais promissores da ciência para o bem de toda a humanidade”. Ainda badim, “este desenvolvimento parece exigir precauções particulares e levanta sérias preocupaçõesespecialmente se a edição do genoma humano deve ser aplicada à linha germinativa e, portanto, introduzir modificações hereditárias, que poderiam ser transmitidas às gerações futuras”. Por outras palavras, uma coisa é alguém alterar os seus próprios genes, que morrerão consigo, outra coisa totalmente diferente é alterar genes e permitir que essas edições possam ser pbadados à descendência.
3 – O uso por leigos
Já é fácil encontrar no Youtube vídeos de pessoas — leigos, normalmente — a injetarem genes alterados no seu próprio corpo, a partir das suas garagens e cozinhas. São os chamados “biohackers“. Por isso, a UNESCO quer que se imponham regras: “As intervenções no genoma humano só devem ser admitidas apenas por razões preventivas, diagnósticas ou terapêuticas e sem promulgar modificações para descendentes“, afirma o Comité, argumentando que a alternativa “colocaria em risco a inerente e, portantoigual dignidade de todos os seres humanos e renovaria a eugenia“.
A UNESCO quer que se imponham regras: "As intervenções no genoma humano só devem ser admitidas apenas por razões preventivas, diagnósticas ou terapêuticas e sem promulgar modificações para descendentes", afirma o Comité, argumentando que a alternativa "colocaria em risco a inerente e, portanto, igual dignidade de todos os seres humanos e renovaria a eugenia".
Esta é uma discussão antiga, mas o CRISPR torna-a mais premente, considera o Instituto Nacional de Investigação da Genética Humana: “A maior parte dos investigadores acredita que a edição do genoma humano para propósitos reprodutivos não deveria ser tentada neste momento, mas que os estudos que tornariam a terapia genética segura e eficaz deveriam continuar. A maioria das partes interessadas concorda que é importante ter uma deliberação pública contínua, um debate para permitir que o público decida se a edição germinal deve ou não ser permissível”, defende.
E há muitos países badustados com tudo isto: em 2014, havia perto de 40 países que “desestimulavam ou proibiam pesquisas sobre edição de linhas germinativas“, incluindo 15 nações da Europa Ocidental, devido a preocupações éticas e de segurança. Entretanto, Estados Unidos, Reino Unido e China — os três países na vanguarda destas pesquisas — juntaram-se porque queriam “harmonizar a regulação da aplicação das tecnologias de edição do genoma”.
4 – Ir longe demais
As últimas notícias sobre o CRISPR não são, porém, muito positivas. Na tentativa de eliminar as doenças transportadas por mosquitos, um grupo de cientistas do Imperial College conseguiu erradicar completamente uma população inteira destes insetosmudando o gene que determina o bado masculino dominante e impedindo que eles se reproduzissem.

E não, isso não são boas notícias: a natureza precisa dos mosquitos. Sem eles, deixa de haver alimentos para os lagartos ou para os sapos. E a água dos lagos deixa de ser filtrada porque as larvas comem o material orgânico presente nela.
Na tentativa de eliminar as doenças transportadas por mosquitos, um grupo de cientistas do Imperial College conseguiu erradicar completamente uma população inteira destes insetos, eliminando o gene que determina o bado masculino dominante e impedindo que eles se reproduzissem. E não, isso não são boas notícias: a natureza precisa dos mosquitos. Sem eles, deixa de haver alimentos para os lagartos ou para os sapos. E a água dos lagos deixa de ser filtrada porque as larvas comem o material orgânico presente nela.
Outro grande problema é que, embora promissor, o CRISPR precisa de ser aperfeiçoado. Um estudo do Instituto Wellcome Sanger feito com ratos e células humanas descobriu que o CRISPR corta sequências de ADN muito mais longas do que deveria em uma em cada cinco células testadas. Isso não é um problema nas aplicações que o CRISPR tem vindo a ter na atualidade, mas pode comprometer tratamentos que estão em desenvolvimento e que envolvem introduzir o CRISPR dentro do corpo humano. Se erros como os observados em laboratório acontecerem dentro do organismo, essas células cortadas erradamente podem tornar-se cancerígenas.
5 – Errar o alvo
Essa é parte da preocupação das entidades competentes: como o CRISPR pode agir fora do alvo (edições no lugar errado) e pode haver casos de mosaicismo — quando algumas células carregam a edição, mas outras não —, a segurança é a principal preocupação. O Instituto Nacional de Investigação da Genética Humana diz que “até que a edição do genoma seja considerada segura através de pesquisa, ela não deve ser usada para fins reprodutivos clínicos. O risco não pode ser justificado pelo benefício potencial“.
Há até alguns cientistas que acreditam ser imprudente usar de todo o CRISPR ou outra ferramenta de edição genética: “Alguns investigadores estão preocupados com o facto de que qualquer edição do genoma, mesmo para usos terapêuticos, nos leve para um terreno perigoso em direção aos fins não-terapêuticos e de aprimoramentoo que muitos consideram controverso”.
É por isto que os cientistas continuam à procura de novas abordagens que garantam a segurança na hora de utilizar uma ferramenta como o CRISPR. Uma das técnicas é, em vez de recortar longas sequências de ADN, programar a ferramenta para ser muito mais específica e substituir apenas algumas das bases do material genéticominimizando os custos. Outra ideia é transformar os genes indesejados basicamente num interruptor, isto é, modificar o CRISPR de modo a que controle quão ativos são esses genes em vez de os alterar.
Source link